Do the electrodes in an electrolytic cell have fixed polarity like a battery?
An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox reaction through the application of electrical energy through the electrodes.
Now, the electrodes in an electrolytic cell cannot have fixed polarity like that of a battery.
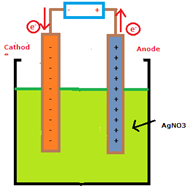
Lets take an example of electrolytic cell as show below:
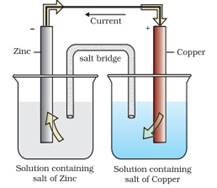
The redox reaction for the above cell is given as below:
Zn(S) +Cu2+ (aq) → Zn3+ (aq) + Cu(s)
The cell potential is 1.1 eV
If external potential less than 1.1 eV is applied to the cell the electron flow from Zn rod to Cu rod hence current flows from Cu to Zn. Zinc rod will act as Cathode while the copper rod will act as Anode.
If the external potential is greater than 1.1V. Electrons flow from Cu to Zn and current flows from Zn to Cu. The Zinc rod act as anode while copper rod acts as cathode.
Thus, we an infer that the electrodes of the electrolytic cell cannot have fixed polarity.