Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Mole fraction is equal to the moles of one component divided by the moles in the solution or mixture.
Given,
Benzene is 30% by the moles in carbon tetrachloride solution.
We get,
In 100g of solution there is 30g of benzene.
∴ Mass of carbon tetrachloride in solution is 70g
Molecular mass of benzene (C6H6) ![]() (6 × 12 + 6 × 1) = 78 g/mol.
(6 × 12 + 6 × 1) = 78 g/mol.
Molecular mass of carbon tetrachloride (CCl4) ![]() (1 × 12 + 4 × 35.5) = 158 g/mol.
(1 × 12 + 4 × 35.5) = 158 g/mol.
∴ Number of moles of benzene (C6H6) ![]()
![]() =
= 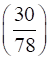 = 0.3846 mol
= 0.3846 mol
Number of moles of carbon tetrachloride (CCl4) = ![]() =
=  = 0.4545 mol
= 0.4545 mol
Thus, Mole fraction of benzene = 
![]()
 = 0.458
= 0.458
∴The mole fraction of benzene = 0.458
24