Calculate the mass of urea (NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
Given,
2.5 kg of 0.25 molal aqueous solution.
Molar mass of urea (NH2CONH2) = (2 (1 × 14 + 2 × 1) + 1 × 12 + 1 × 16)
= 60 g/mol
1000 g of water contains 0.25 mol = (0.25 × 60) g of urea.
= 15 g of urea.
Means, 1015 g of solution contains 15 g of urea
Therefore,
2500 g of solution contains = 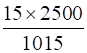
= 36.95 g
Hence, mass of urea required is 37 g (approx).
15