Calculate (a) molality (b) molarity and (c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI is 1.202 g mL-1.
(a) Molality, also called molal concentration, is a measure of the concentration of a solute in a solution in terms of amount of substance in a specified amount of mass of the solvent.
Molar mass of KI ![]() 39 + 127 = 166 g/mol.
39 + 127 = 166 g/mol.
20% aqueous solution of KI means 200 g of KI is present in 1000 g of solution.
Therefore,
Molality = 
= 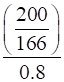
= 1.506 m
∴ Molality of KI = 1.506 m
(b) Molarity is the concentration of a solution expressed as the number of moles of solute per litre of solution.
Given,
Density of the solution = 1.202 g/mL
Volume of 100 g solution = 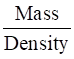
= 
= 83.19 mL
Therefore, molarity = 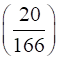 mol/ 83.19 × 10 - 3 L
mol/ 83.19 × 10 - 3 L
= 1.45 M
∴ Molarity of KI = 1.45M
(c) Molar mass of KI ![]() 39 + 127 = 166 g/mol.
39 + 127 = 166 g/mol.
Moles of KI ![]()
 = 0.12 mol
= 0.12 mol
Moles of water = 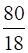 = 4.44 mol
= 4.44 mol
Therefore,
Mole fraction of KI = 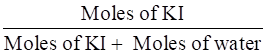
= 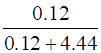
= 0.0263
∴ Mole fraction of KI = 0.0263