Vapour pressure of pure water at 298 K is 23.8 mm Hg. 50 g of urea (NH2CONH2) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Given,
Vapour pressure of water, PIo = 23.8 mm of Hg
Weight of water, w1 = 850 g
Weight of urea, w2 = 50 g
Molecular weight of water, M1 = 18 g/mol
Molecular weight of urea, M2 = 60 g/mol
n1 = 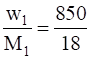 = 47.22 mol
= 47.22 mol
n2 =  = 0.83 mol
= 0.83 mol
We have to calculate vapour pressure of water in the solution p1
By using Rault's therom,
![]()

![]()

![]()
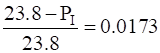 (relative lowering)
(relative lowering)
![]() PI = 23.4 mm of Hg
PI = 23.4 mm of Hg
Hence,
The vapour pressure of water in the solution is 23.4mm of Hg and its relative lowering is 0.0173.
14