Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.
Given,
Mass of water, wl = 500 g
Boiling point of water = 99.63°C (at 750 mm Hg).
Molal elevation constant, Kb = 0.52 K kg/mol
Molar mass of sucrose (C12H22O11), M2 ![]() (11 × 12 + 22 × 1 + 11 × 16) = 342 g/mol
(11 × 12 + 22 × 1 + 11 × 16) = 342 g/mol
Elevation of boiling point ΔTb = (100 + 273) - (99.63 + 273) = 0.37 K
We know that,
ΔTb = 
0.37 = 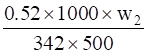
w2 = 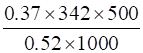
w2 = 121.67 g
Hence,
121.67 g (approx) Sucrose is added to 500g of water so that it boils at 100°C.
12