Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5°C. Kf = 3.9 K kg mol-1.
Given,
Mass of acetic acid, w1 = 75 g
Lowering of melting point, ΔTf = 1.5 K
Kf = 3.9 K kg/mol
Molar mass of ascorbic acid (C6H8O6), M2![]() 6 × 12 + 8 × 1 + 6 × 16 = 176 g/mol[1]
6 × 12 + 8 × 1 + 6 × 16 = 176 g/mol[1]
We know that,
ΔTb = 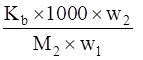
1.5 = 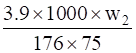
w2 = 
= 5.08 g
Hence,
5.08 g of ascorbic acid is needed to be dissolved.
12