Write the mechanism of the reaction of HI with methoxymethane.
The reaction of HI with methoxymethane yields two different sets of products depending upon the initial amount of HI taken.
(i) When equal moles of HI and methoxymethane are taken, a mixture of methyl alcohol and methyl iodide is formed.
The mechanism is given below:
In the first step, methoxymethane reacts with hydrogen iodide to extract a proton to give the dimethyloxonium ion.
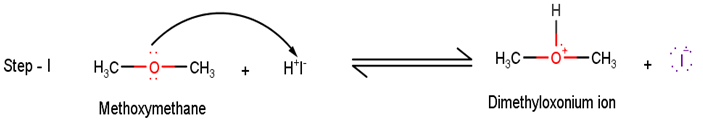
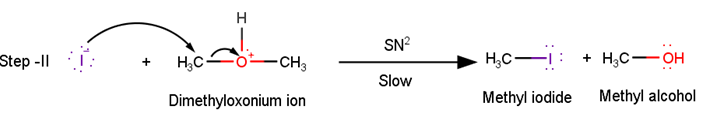
In the second step of the reaction, the Dimethyloxonium ion reacts with the iodide ion present to yield methyl iodide and methyl alcohol as the product via SN2 pathway.

(ii) If an excess of HI is used the methyl alcohol formed in Step II is also converted into methyl iodide by the following mechanism : -
In the first step, methoxymethane reacts with hydrogen iodide to extract a proton to give the dimethyloxonium ion.

In the second step of the reaction the Dimethyloxonium ion reacts with the iodide ion present to yield methyl iodide and methyl alcohol as the product via SN2 pathway.
In the third step of the reaction Methyl alcohol formed above reacts with hydrogen iodide to extract a proton to give the protonated methyl alcohol which finally reacts in the fourth step with the iodide ion via SN2 pathway to give methyl iodide and water as the product.
