Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively. Given that molecular mass is 159.69g.
Given:
Percentage of Iron in the oxide = 69.9%
Percentage of oxygen in the oxide = 30.1%
Finding Empirical Formula:
To determine the empirical formula of iron oxide, we need to find the simplest whole number ratio.
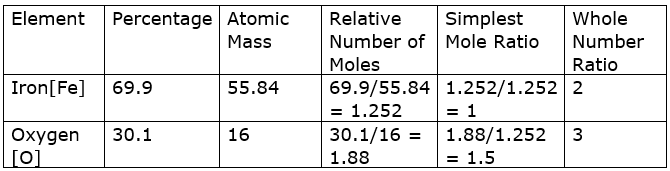
Therefore, the empirical formula for iron oxide is Fe2O3.
Finding Molecular Formula:
Empirical Formula Mass = [55.84×2] + [16×3]
= 111.68 + 48
= 159.68g
Molecular Mass [given] = 159.69g
n = [Molecular Mass]/ [Empirical Formula Mass]
= 159.69/159.68
= 1
Molecular Formula = n × Empirical Formula
= 1 × Fe2O3.
= Fe2O3
Therefore, the Molecular Formula is Fe2O3.
11