AIM
To perform and observe the reaction of iron nails kept in copper sulphate solution and classify the reaction.
MATERIALS REQUIRED
Apparatus: Test tubes, test tube stand, sandpaper, iron nails, and thread.
Chemical compound: Copper sulphate solution
THEORY
1. On immersion iron nail inside the copper sulphate solution, iron being more reactive displaces the copper from its salt solution.
2. The initial colour of the copper sulphate solution changes from blue which after adding iron nail changes to light green.
3. Iron (Fe2+) ions replace the copper ions (Cu2+) from the solution and form iron sulphate. Hence it is called a Displacement reaction.
The chemical reaction for the above chemical change is given as follow:
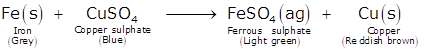


PROCEDURE
1. Take two iron nails and remove the iron oxide layer using sandpaper formed due to oxidation of iron with oxygen in the air.
2. Take two clean, dry test tubes and mark them as ‘A’ and ‘B’ using a marker.
3. In each test tube pour 10 ml of freshly prepared copper sulphate solution.
4. Tie one iron nail using a thread and immerse it in the copper sulphate solution in test tube A in the rest state for 30 minutes and keep aside the second nail to measure the change in the first iron nail.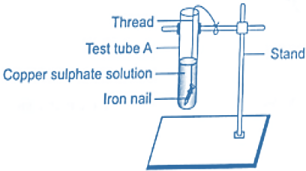
5. After 30 minutes elapsed, take out the immersed iron nail from the copper sulphate solution and compare the colour of copper sulphate solutions in the test tubes ‘A’ and ‘B’ Also compare the colour of iron nail with one kept aside for testing purpose.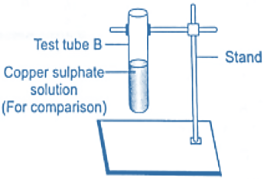
OBSERVATIONS
The primary colour of copper sulphate solution is blue which after immersing iron nails get changed to light green.
After immersing nail into copper sulphate solution, a brown coat develops over the surface of the iron nail. Because of this coat of copper oxide on the surface of iron nail clour get changed to brown colour.
RESULT
On Immersing the iron nails into the copper sulphate solution, displacement reaction takes place.
Iron being more reactive than copper displaces the copper ions from its salt solution, and two new products ferrous sulphate and the pure copper in solid is formed as the product of the given chemical reaction.
PRECAUTIONS
1. The iron nails should be cleaned using sandpaper.
2. The iron nail must be completely immersed in the copper sulphate solution.
3. Do not touch copper sulphate or the iron nail dipped in that solution as it can disturb the reaction.