AIM
To study or the following properties or acetic acid (ethanoic acid):
(i) Odour
(ii) Solubility in water
(iii) Effect on litmus
(iv) Reaction with sodium hydrogen carbonate
MATERIALS REQUIRED
Glass rod, acetic acid, litmus paper, sodium bicarbonate, test tubes, water, test tube stand, lime water.
THEORY
(i) Acetic acid is an organic acid, its 5-8% concentrated solution is called vinegar.
(ii) It is a weak acid. It ionises partially in presence of water as shown below:
CH3COOH + H2O ⇋ CH3COO-(aq) + H2O+(aq)
(iii) Acetic acid has pH <7, hence It turns blue litmus red.
(iv) It is completely soluble in water.
(v) It reacts with sodium bicarbonate to liberate CO2, which turns lime water milky and the milkiness disappears if an excess of CO2 is passed through the solution.
![]()
![]()
![]()
PROCEDURE
1. Odour
Take a small amount of acetic acid in a test tube.
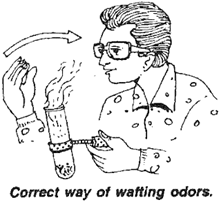
2. Solubility in water
Take 2 mL of water in a test tube and add a few drops of acetic acid. Shake the test tube carefully.
3. Effect on litmus
Take a strip of blue litmus paper and add one drop of acetic solution on the blue litmus paper.
Take a red litmus paper and add one drop of acetic acids solution on red litmus paper.
4. Reaction with sodium bicarbonate
Take 5 mL of acetic acid in a test tube and add sodium bicarbonate.
Pass the evolving gas through lime water.

OBSERVATIONS
S.No. |
Observation |
Inference |
1. |
The smell of vinegar is pungent. |
Acetic acid has an irritating and pungent smell. |
2. |
A homogeneous solution is obtained. |
Acetic acid is soluble in water in all proportions. |
3. |
Blue litmus turns red. There is no change in the colour of red litmus. |
Acetic acid is acidic in nature. Acetic acid is not basic in nature. |
4. |
A colourless, odourless gas with brisk effervescence is evolved. Lime water turns milky. |
Acetic acid reacts with sodium bicarbonate, and gas is evolved. The gas evolved is CO2. |
RESULT
1. Acetic acid has a pungent odour.
2. Acetic acid is completely soluble in water i.e. acetic acid and water form a true solution.
3. Acetic acid having a pH <7 turns blue litmus paper red.
4. Acetic acid does not change the colour of blue litmus paper.
5. CO2 gas is liberated when it reacts with sodium bicarbonate.
PRECAUTIONS
1. Do not inhale vapours of acetic acid directly.
2. Ethanoic acid should be handled with care.
3. Freshly prepared lime water solution should be used.