Table of Contents
5
Separation of Substances
In our daily life, there are many instances when we notice a substance being separated from a mixture of materials.
Tea leaves are separated from the liquid with a strainer, while preparing tea (Fig. 5.1).
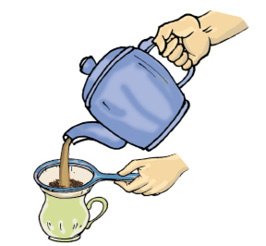
Fig. 5.1 Separating tea leaves with a strainer
Grain is separated from stalks, while harvesting. Milk or curd is churned to separate the butter (Fig. 5.2). As we learned in Chapter 3, we gin cotton to separate its seeds from the fibre.
Perhaps you might have eaten salted daliya or poha. If you found that it had chillies in it, you may have carefully taken them out before eating.
Suppose you are given a basket containing mangoes and guavas and asked to separate them. What would you do? Pick out one kind and place them in a separate container, right?
Seems easy, but what if the materials we want to separate are much smaller than mango or guava? Imagine you are given a glass of sand with salt mixed in it. Impossible, even to think of separating salt from this mixture by picking out grains of sand by hand!
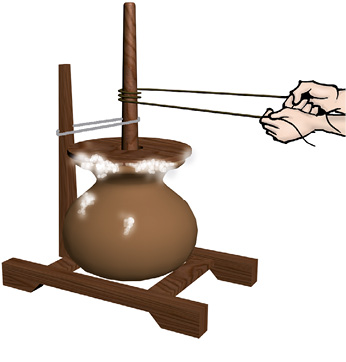
Fig. 5.2 Butter is taken out by churning milk or curd

Activity 1
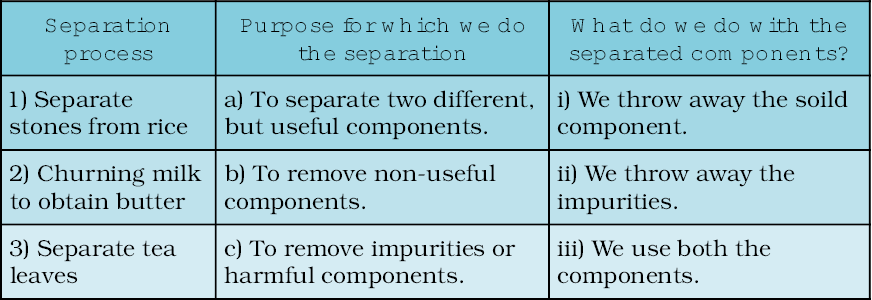
In Column 1 of Table 5.1, are given a few processes of separation. The purpose of separation and the way separated components are used is mentioned in Column 2 and 3 respectively. However, the information given in Columns 2 and 3 is jumbled up. Can you match each process with its purpose and the way separated components are used?
Table 5.1 Why do we separate substances?
We see that, before we use a substance, we need to separate harmful or non-useful substances that may be mixed with it. Sometimes, we separate even useful components if we need to use them separately.
The substances to be separated may be particles of different sizes or materials. These may be in any three states of matter i.e., solid, liquid or gas. So, how do we separate substances mixed together if they have so many different properties?
5.1 Methods of Separation
We will discuss some simple methods of separating substances that are mixed together. You may come across some of these methods being used in day to day activities.
Handpicking
Activity 2
Bring a packet of food grain purchased from a shop to the classroom. Now, spread the grains on a sheet of paper. Do you find only one kind of grain on the sheet of paper? Are there pieces of stone, husks, broken grain and particles of any other grain in it? Now, remove with your hand the pieces of stone, husks and other grains from it.
This method of handpicking can be used for separating slightly larger sized impurities like the pieces of dirt, stone, and husk from wheat, rice or pulses (Fig. 5.3). The quantity of such impurities is usually not very large. In such situations, we find that handpicking is a convenient method of separating substances.
Threshing
You must have seen bundles of wheat or paddy stalks lying in fields after harvesting the crop. Stalks are dried in the sun before the grain is separated from them. Each stalk has many grain seeds attached to it. Imagine the number of grain seeds in hundreds of bundles of stalk lying in the field! How does the farmer separate grain seeds from those bundles of stalks?
One may pluck mangoes or guavas from the trees. But, grain seeds are much smaller than mangoes or guavas. So, plucking them from their stalks would be impossible. How does one separate grain seeds from their stalks?

Fig. 5.4 Threshing
The process that is used to separate grain from stalks etc. is threshing. In this process, the stalks are beaten to free the grain seeds (Fig. 5.4). Sometimes, threshing is done with the help of bullocks. Machines are also used to thresh large quantities of grain.
Winnowing
Activity 3
Make a mixture of dry sand with sawdust or powdered dry leaves. Keep this mixture on a plate or a newspaper. Look at this mixture carefully. Can the two different components be made out easily? Are the sizes of particles of the two components similar? Would it be possible to separate the components by handpicking?
Now, take your mixture to an open ground and stand on a raised platform. Put the mixture in a plate or sheet of paper. Hold the plate or the sheet of paper containing the mixture, at your shoulder height. Tilt it slightly, so that the mixture slides out slowly.
What happens? Do both the components — sand and sawdust (or powdered leaves) fall at the same place? Is there a component that blows away? Did the wind manage to separate the two components?
This method of separating components of a mixture is called winnowing. Winnowing is used to separate heavier and lighter components of a mixture by wind or by blowing air. This method is commonly used by farmers to separate lighter husk particles from heavier seeds of grain (Fig. 5.5).
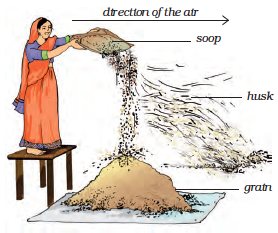
The husk particles are carried away by the wind. The seeds of grain get separated and form a heap near the platform for winnowing. The separated husk is used for many purposes such as fodder for cattles.
Sieving
Sometimes, we may wish to prepare a dish with flour. We need to remove impurities and bran that may be present in it. What do we do? We use a sieve and pour the flour into it (Fig. 5.6).
Sieving allows the fine flour particles to pass through the holes of the sieve while the bigger impurities remain on the sieve.
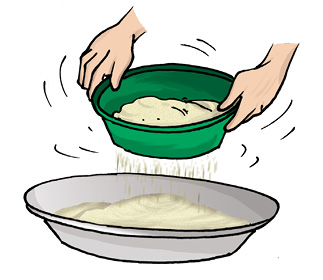
Fig. 5.6 Sieving
In a flour mill, impurities like husk and stones are removed from wheat before grinding it. Usually, a bagful of wheat is poured on a slanting sieve. The sieving removes pieces of stones, stalk and husk that may still remain with wheat after threshing and winnowing.

Fig. 5.7 Pebbles and stones are removed from sand by sieving
You may have also noticed similar sieves being used at construction sites to separate pebbles and stones from sand (Fig. 5.7).
Activity 4
Bring a sieve and a small quantity of flour from home, to the class. Sieve the flour to separate any impurities in it. Now, make a fine powder of chalk pieces and mix it with the flour. Can we separate the flour and the powdered chalk by sieving?
Sieving is used when components of a mixture have different sizes.
Sedimentation, Decantation and Filtration
Sometimes, it may not be possible to separate components of a mixture by winnowing and handpicking. For example, there may be lighter impurities like dust or soil particles in rice or pulses. How are such impurities separated from rice or pulses before cooking?
Rice or pulses are usually washed before cooking. When you add water to these, the impurities like dust particles get separated. These impurities go into water. Now, what will sink to the bottom of the vessel — rice or dust? Why? Have you seen that the vessel is tilted to pour out the dirty water?
When the heavier component in a mixture settles after water is added to it, the process is called sedimentation. When the water (along with the dust) is removed, the process is called decantation (Fig. 5.8). Let us find a few other mixtures that can be separated through sedimentation and decantation.
The same principle is used for separating a mixture of two liquids that do not mix with each other. For example, oil and water from their mixture can be separated by this process. If a mixture of such liquids is allowed to stand for some time, they form two separate layers. The component that forms the top layer can then be separated by decantation.
Let us again consider a mixure of a solid and liquid. After preparing tea, what do you do to remove the tea leaves? Usually, we use stainer to remove tea leaves. Try decantation. It helps a little. But, do you still get a few leaves in your tea? Now, pour the tea through a strainer. Did all the tea leaves remain in the strainer? This process is called filtration (Fig. 5.1). Which method of separating tea leaves from prepared tea is better, decantation or filtration?
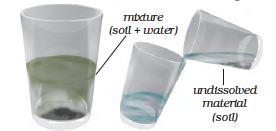
Fig. 5.8 Separating two components of a mixture by sedimentation and decantation
Activity 5
Collect some muddy water from a pond or a river. If it is not available, mix some soil to water in a glass. Let it stand for half an hour. Observe the water carefully and note your observations.
Does some soil settle at the bottom of water? Why? What will you call this process?
Now, slightly tilt the glass without disturbing the water. Let the water from the top flow into another glass (Fig. 5.8). What will you call this process?
Is the water in the second glass still muddy or brown in colour? Now filter it. Did the tea strainer work? Let us try filtering the water through a piece of cloth. In a piece of cloth, small holes or pores remain in between the woven threads. These pores in a cloth can be used as a filter.
If the water is still muddy, impurities can be separated by a filter that has even smaller pores. A filter paper is one such filter that has very fine pores in it. Fig. 5.9 shows the steps involved in using a filter paper. A filter paper folded in the form of a cone is fixed onto a funnel (Fig. 5.10). The mixture is then poured on the filter paper. Solid particles in the mixture do not pass through it and remain on the filter.
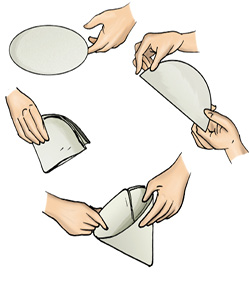

Fig. 5.10 Filtration using a filter paper
Evaporation
Activity 6
Add two spoons of salt to water in another beaker and stir it well. Do you see any change in the colour of water? Can you see any salt in the beaker, after stirring? Heat the beaker containing the salt water (Fig. 5.11). Let the water boil away. What is left in the beaker?

Fig. 5.11 Heating a beaker containing salt water
In this activity, we used the process of evaporation, to separate a mixture of water and salt.
The process of conversion of water into its vapour is called evaporation. Theprocess of evaporation takes place continuously wherever water is present.
Where do you think, salt comes from? Sea water contains many salts mixed in it. One of these salts is the common salt. When sea water is allowed to stand in shallow pits, water gets heated by sunlight and slowly turns into water vapour, through evaporation. In a few days, the water evaporates completely leaving behind the solid salts (Fig. 5.12). Common salt is then obtained from this mixture of salts by further purification.

Fig. 5.12 Obtaining salt from sea water
Use of more than one method of separation
We have studied some methods for separation of substances from their mixtures. Often, one method is not sufficient to separate the different substances present in a mixture. In such a situation, we need to use more than one of these methods.
Activity 7
Take a mixture of sand and salt. How will we separate these? We already saw that handpicking would not be a practical method for separating these.
Keep this mixture in a beaker and add some water to it. Leave the beaker aside for some time. Do you see the sand settling down at the bottom? The sand can be separated by decantation or filtration. What does the decanted liquid contain? Do you think this water contains the salt which was there in the mixture at the beginning?
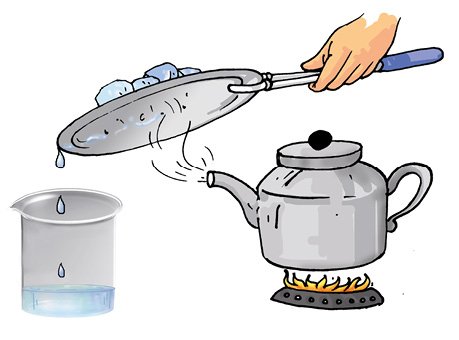
Now, we need to separate salt and water from the decanted liquid. Transfer this liquid to a kettle and close its lid. Heat the kettle for some time. Do you notice steam coming out from the spout of the kettle?
Take a metal plate with some ice on it. Hold the plate just above the spout of the kettle as shown in Fig. 5.13. What do you observe? Let all the water in the kettle boil off.
When the steam comes in contact with the metal plate cooled with ice, it condenses and forms liquid water. The water drops that you observed falling from the plate, were due to condensation of steam. The process of conversion of water vapour into its liquid form is called condensation.
Did you ever see water drops condensed under a plate that has been used to cover a vessel containing milk that has just been boiled?
After all the water has evaporated, what is left behind in the kettle?
We have thus, separated salt, sand and water using processes of decantation, filtration, evaporation and condensation.
Paheli faced a problem while recovering salt mixed with sand. She has mixed a packet of salt in a small amount of sand. She then tried the method suggested in Activity 7, to recover the salt. She found, however, that she could recover only a small part of the salt that she had taken. What could have gone wrong?
Can water dissolve any amount of a substance?
In chapter 4, we found that many substances dissolve in water and form a solution. We say that these substances are soluble in water. What will happen if we go on adding more and more of these substances to a fixed quantity of water?
Activity 8
You will need a beaker or a small pan, a spoon, salt and water. Pour half a cup of water in the beaker. Add one teaspoonful of salt and stir it well, until the salt dissolves completely (Fig 5.14). Again add a teaspoonful of salt and stir well. Go on adding salt, one teaspoonful at a time, and stir.
After adding a few spoons of salt, do you find that some salt remains undissolved and settles at the bottom of the beaker? If yes, this means that no more salt can be dissolved in the amount of water we have taken. The solution is now said to be saturated.
Here is a hint as to what might have gone wrong when Paheli tried to recover large quantity of salt mixed with sand. Perhaps the quantity of salt was much more than that required to form a saturated solution. The undissolved salt would have remained mixed with the sand and could not be recovered. She could solve her problem by using a larger quantity of water.
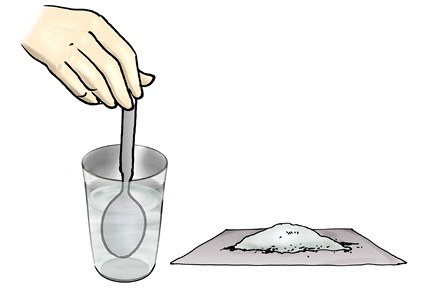
Fig 5.14 Dissolving salt in water
Suppose, she did not have sufficient quantity of water to dissolve all the salt in the mixture. Is there some way that water could be made to dissolve more salt before the solution gets saturated?
Let us try and help Paheli out.
Activity 9
Take some water in a beaker and mix salt in it until it cannot dissolve any more salt. This will give you a saturated solution of salt in water.
Now, add a small quantity of salt to this saturated solution and heat it. What do you find? What happens to the undissolved salt in the bottom of the beaker? Does it dissolve, now? If yes, can some more salt be dissolved in this solution by heating it?
Let this hot solution cool. Does the salt appear to settle at the bottom of the beaker again?
The activity suggests that larger quantity of salt can be dissolved in water on heating.
Does water dissolve equal amounts of different soluble substances? Let us find out.
Activity 10
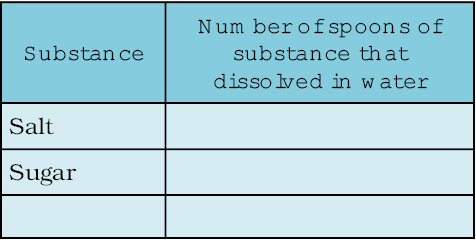
Take two glasses and pour half a cup of water in each of them. Add a teaspoon of salt to one glass and stir till the salt dissolves. Go on adding salt, one teaspoon at a time, till the solution saturates. Record the number of spoons of salt that dissolved in the water, in Table 5.2. Now, repeat the same activity with sugar. Repeat this with some other substances that are soluble in water.
What do you notice from Table 5.2? Do you find that water dissolves different substances in different amounts?
Table 5.2
We have discussed a few methods of separating substances. Some of the methods of separation presented in this chapter are also used in a science laboratory.
We also learnt that a solution is prepared by dissolving a substance in a liquid. A solution is said to be saturated if it cannot dissolve more of the substance in it.
KeyWords

Churning
Condensation
Evaporation
Filtration
Handpicking
Saturated solution
sedimentation
Sieving
Solution
Threshing
Winnowing
Summary
- Handpicking, winnowing, sieving, sedimentation, decantation and filtration are some of the methods of separating substances from their mixtures.
- Husk and stones could be separated from grains by handpicking.
- Husk is separated from heavier seeds of grain by winnowing.
- Difference in the size of particles in a mixture is utilised to separate them by the process of sieving and filtration.
- In a mixture of sand and water, the heavier sand particles settle down at the bottom and the water can be separated by decantation.
- Filtration can be used to separate components of a mixture of an insoluble solid and a liquid.
- Evaporation is the process in which a liquid gets converted into its vapour. Evaporation can be used to separate a solid dissolved in a liquid.
- A saturated solution is one in which no more of that substance can be dissolved.
- More of a substance can be dissolved in a solution by heating it.
- Water dissolves different amount of soluble substances in it.
Exercises
1. Why do we need to separate different components of a mixture? Give two examples.
2. What is winnowing? Where is it used?
3. How will you separate husk or dirt particles from a given sample of pulses before cooking.
4. What is sieving? Where is it used?
5. How will you separate sand and water from their mixture?
6. Is it possible to separate sugar mixed with wheat flour? If yes, how will you do it?
7. How would you obtain clear water from a sample of muddy water?
8. Fill up the blanks
(a) The method of separating seeds of paddy from its stalks is called ___________.
(b) When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of ___________.
(c) Salt is obtained from seawater by the process of ___________.
(d) Impurities settled at the bottom when muddy water was kept overnight in a bucket. The clear water was then poured off from the top. The process of separation used in this example is called ___________.
9. True or false?
(a) A mixture of milk and water can be separated by filtration.
(b) A mixture of powdered salt and sugar can be separated by the process of winnowing.
(c) Separation of sugar from tea can be done with filtration.
(d) Grain and husk can be separated with the process of decantation.
10. Lemonade is prepared by mixing lemon juice and sugar in water. You wish to add ice to cool it. Should you add ice to the lemonade before or after dissolving sugar? In which case would it be possible to dissolve more sugar?
suggested Projects and Activities
1. Visit a nearby dairy and report about the processes used to separate cream from milk.
2. You have tried a number of methods to separate impurities like mud from water. Sometimes, the water obtained after employing all these processes could still be a little muddy. Let us see if we can remove even this impurity completely. Take this filtered water in a glass. Tie a thread to a small piece of alum. Suspend the piece of alum in the water and swirl. Did the water become clear? What happened to the mud? This process is called loading. Talk to some elders in your family to find out whether they have seen or used this process.
Things to see

“The winnowers”, painted by Gustav Courbet in 1853
Reproduced with permission from Museè de Beaus Arts, Nantes, France
