Table of Contents

5. Acids, Bases and Salts
We use in our daily life a large
number of substances such
as lemon, tamarind, common salt, sugar and vinegar. Do they have the same taste? Let us recall tastes of some edible substances listed in
Table 5.1. If you have not tasted any of these substances taste it now and enter the result in Table 5.1.
| CAUTION |
|
| Student | Taste (sour/bitter/any other) |
| Lemon juice | |
| Orange juice | |
| Vinegar | |
| Curd | |
| Tamarind (imli) | |
| Sugar | |
| Common salt | |
| Amla | |
| Baking soda | |
| Grapes | |
| Unripe mango | |
| Cucumber |
5.1 Acids and Bases
Curd, lemon juice, orange juice and vinegar taste sour. These substances taste sour because they contain acids. The chemical nature of such substances is acidic. The word acid comes from the Latin word acere which means sour. The acids in these substances are natural acids.
What about baking soda? Does it also taste sour? If not, what is its taste? Since, it does not taste sour it means, that it has no acids in it. It is bitter in taste. If you rub its solution between fingers, it feels soapy. Generally, substances like these which are bitter in taste and feel soapy on touching are known as bases. The nature of such substances is said to be basic.
If we cannot taste every substance, how do we find its nature?
Special type of substances are used to test whether a substance is acidic or basic. These substances are known as indicators. The indicators change their colour when added to a solution containing an acidic or a basic substance. Turmeric, litmus, China rose petals (Gudhal), etc., are some of the naturally occurring indicators.

5.2 Natural Indicators Around Us
Litmus: A natural dye
The most commonly used natural indicator is litmus. It is extracted from lichens (Fig. 5.1a). It has a mauve (purple) colour in distilled water. When added to an acidic solution, it turns red and when added to a basic solution, it turns blue. It is available in the form of a solution, or in the form of strips of paper, known as litmus paper. Generally, it is available as red and blue litmus paper (Fig. 5.1b).
(a)


(b)
Activity 5.1
- Mix some water with lemon juice in a plastic cup/tumbler/test tube.
- Put a drop of the above solution on a strip of the red litmus paper with the help of a dropper.
Is there any change in colour? - Repeat the same exercise with the blue litmus paper.
Note down if there is any change in colour.
Perform the same activity with the following substances:
Tap water, detergent solution, aerated drink, soap solution, shampoo, common salt solution, sugar solution, vinegar, baking soda solution, milk of magnesia, washing soda solution, lime water. If possible make solutions in distilled water.
Record your observations as in Table. 5.2.
In your Table, are there any substances on which litmus had no effect? Name those substances.
The solutions which do not change the colour of either red or blue litmus are known as neutral solutions. These substances are neither acidic nor basic.

Turmeric is another natural indicator
Activity 5.2
- Take a tablespoonful of turmeric powder. Add a little water and make a paste.
- Make turmeric paper by depositing turmeric paste on blotting paper/filter paper and drying it. Cut thin strips of the yellow paper obtained.
- Put a drop of soap solution on the strip of turmeric paper.
What do you observe?
| S. No. | Test solution | Effect on red litmus paper | Effect on blue litmus paper | Inference |
You can prepare a greeting card for your mother on her birthday. Apply turmeric paste on a sheet of plane white paper and dry it. Draw a beautiful flower with soap solution with the help of a cotton bud. You will get a beautiful greeting card.  |
Similarly test the solutions listed in Table 5.3 and note down your observations. You may try solutions of other substances also.
China Rose as Indicator
Activity 5.3
Collect some china rose (Gudhal) petals and place them in a beaker. Add some warm water. Keep the mixture for some time till water becomes coloured. Use the coloured water as an indicator. Add five drops of the indicator to each of the solutions given in Table 5.4.

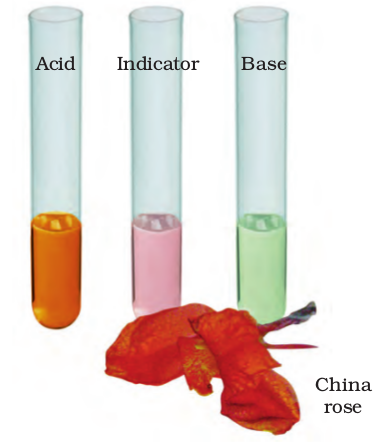
Fig. 5.3 China rose flower and indicator prepared from it
| S. No. | Test solution | Effect on turmeric solution | Remarks |
| 1. | Lemon juice | ||
| 2. | Orange juice | ||
| 3. | Vinegar | ||
| 4. | Milk of magnesia | ||
| 5. | Baking soda | ||
| 6. | Lime water | ||
| 7. | Sugar | ||
| 8. | Common salt |
| S. No. | Test solution | Initial Colour | Final Colour |
| 1. | Shampoo (dilute solution) | ||
| 2. | Lemon juice | ||
| 3. | Soda water | ||
| 4. | Sodium hydrogencarbonate solution | ||
| 5. | Vinegar | ||
| 6. | Sugar solution | ||
| 7. | Common salt solution |
What is the effect of the indicator on acidic, basic and neutral solutions? China rose indicator (Fig. 5.3) turns acidic solutions to dark pink (magenta) and basic solutions to green.

Paheli brought the following paheli (riddle) for you.
Coffee is brown And bitter in taste. Is it an acid? Or a base? Don’t give the answer Without any test, You are in the dark With its taste. |
Activity 5.4
The teacher is requested to get the dilute solution of the following chemicals from his/her school laboratory or from a nearby school: hydrochloric acid, sulphuric acid, nitric acid, acetic acid, sodium hydroxide, ammonium hydro-xide, calcium hydroxide (lime water). Demonstrate the effect of the three indicators on each of these solutions. Record your observations in Table 5.5.
| S. No. | Name of acid | Effect on litmus paper | Effect on turmeric paper | Effect on China rose solution |
| 1. | Dilute hydrochloric acid | |||
| 2. | ||||
| 3. |
Are you familiar with the term acid rain? Have you ever heard about damaging effect of acid rain? As the name indicates the rain containing excess of acids is called an acid rain. Where do these acids come from? The rain becomes acidic because carbon dioxide, sulphur dioxide and nitrogen dioxide (which are released into the air as pollutants) dissolve in rain drops to form carbonic acid, sulphuric acid and nitric acid respectively. Acid rain can cause damage to buildings, historical monuments, plants and animals. |
| CAUTION |
| Great care should be taken while handling laboratory acids and bases because these are corrosive in nature, irritating and harmful to skin. |
5.3 Neutralisation
Fill one fourth of a test tube with dilute hydrochloric acid. Note down its colour. Note down the colour of phenolphthalein solution also. Add 2–3 drops of the indicator to the acid. Now shake the test tube gently. Do you observe any change in colour of the acid?
It is evident that when the solution is basic, phenolphthalein gives a pink colour. On the other hand, when the solution is acidic, it remains colourless.

When an acidic solution is mixed with a basic solution, both the solutions neutralise the effect of each other. When an acid solution and a base solution are mixed in suitable amounts, both the acidic nature of the acid and the basic nature of the base are destroyed. The resulting solution is neither acidic nor basic. Touch the test tube immediately after neutralisation. What do you observe? In neutralisation reaction, heat is always produced, or evolved. The evolved heat raises the temperature of the reaction mixture.
In neutralisation reaction a new substance is formed. This is called salt. Salt may be acidic, basic or neutral in nature. Thus, neutralisation can be defined as follows:
The reaction between an acid and a base is known as neutralisation. Salt and water are produced in this process with the evolution of heat.
The following reaction is an example:
Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) →Sodium chloride (NaCl) + Water (H2O)
Boojho added dilute sulphuric acid to lime water. Will the reaction mixture become hot or cool?
5.4 Neutralisation in Everyday Life
Indigestion
Our stomach contains hydrochloric acid. It helps us to digest food, as you have learnt in Chapter 2. But too
much of acid in the stomach causes indigestion. Sometimes indigestion is painful. To relieve indigestion, we take an antacid such as milk of magnesia, which contains magnesium hydroxide. It neutralises the effect of excessive acid.
Ant bite
When an ant bites, it injects the acidic liquid (formic acid) into the skin. The effect of the acid can be neutralised by rubbing moist baking soda (sodium hydrogencarbonate) or calamine solution, which contains zinc carbonate.
Soil treatment
Excessive use of chemical fertilisers makes the soil acidic. Plants do not grow well when the soil is either too acidic or too basic. When the soil is too acidic, it is treated with bases like quick lime (calcium oxide) or slaked lime (calcium hydroxide). If the soil is basic, organic matter (compost) is added to it. Organic matter releases acids which neutralises the basic nature of the soil.
Factory wastes
The wastes of many factories contain acids. If they are allowed to flow into the water bodies, the acids
will kill fish and other organisms. The factory wastes are, therefore, neutralised by adding basic
substances.
| Keywords | ||
| Acid | Basic | Neutralisation |
| Acidic | Indicator | Salt |
| Base | Neutral |
| What you have learnt |
|
Exercises
1. State differences between acids and bases.
2. Ammonia is found in many household products, such as window cleaners. It turns red litmus blue. what is its nature?
3. Name the source from which litmus solution is obtained. What is the use of this solution?
4. Is the distilled water acidic/basic/neutral? How would you verify it?
5. Describe the process of neutralisation with the help of an example.
6. Mark ‘T’ if the statement is true and ‘F’ if it is false:
(i) Nitric acid turn red litmus blue. (T/F)
(ii) Sodium hydroxide turns blue litmus red. (T/F)
(iii) Sodium hydroxide and hydrochloric acid neutralise each other and form salt and water. (T/F)
(iv) Indicator is a substance which shows different colours in acidic and basic solutions. (T/F)
(v) Tooth decay is caused by the presence of a base. (T/F)
7. Dorji has a few bottles of soft drink in his restaurant. But, unfortunately, these are not labelled. He has to serve the drinks on the demand of customers. One customer wants acidic drink, another wants basic and third one wants neutral drink. How will Dorji decide which drink is to be served to whom?
8. Explain why:
(a) An antacid tablet is taken when you suffer from acidity.
(b) Calamine solution is applied on the skin when an ant bites.
(c) Factory waste is neutralised before disposing it into the water bodies.
9. Three liquids are given to you. One is hydrochloric acid, another is sodium hydroxide and third is a sugar solution. How will you identify them? You have only turmeric indicator.
10. Blue litmus paper is dipped in a solution. It remains blue. What is the nature of the solution? Explain.
11. Consider the following statements:
(a) Both acids and bases change colour of all indicators.
(b) If an indicator gives a colour change with an acid, it does not give a change with a base.
(c) If an indicator changes colour with a base, it does not change colour with an acid.
(d) Change of colour in an acid and a base depends on the type of the indicator.
Which of these statements are correct?
(i) All four (ii) a and d (iii) b, c and d (iv) only d
Extended learning — Activities and Projects
1. Using the knowledge of acids and bases, write a secret message with the help of baking soda and beet root. Explain how it works.
(Hint: Prepare baking soda solution in water. Use this solution to write the message on a sheet of white paper with a cotton bud. Rub a slice of fresh beet root over the message.)
2. Prepare red cabbage juice by boiling a piece of red cabbage in water. Use it as an indicator and test the acidic and basic solutions with it. Present your observations in the form of a table.
3. Bring the soil sample of your area, find out if it is acidic, basic or neutral. Discuss with farmers if they treat the soil in any manner.
4. Visit a doctor. Find out the medicines, he prescribes to treat acidity. Ask him how acidity can be prevented.
| Did you know? |
Each cell in our body contains an acid, the deoxyribonucleic acid or DNA. It controls every feature of the body such as our looks, colour of our eyes, our height etc. Proteins that build part of our cells are also made of amino acids. The fats in our body contain fatty acids. |