Table of Contents

Materials : Metals and Non-Metals
You are familiar with a number of materials like iron, aluminium, copper, etc. Some materials have been given in Table 4.1.
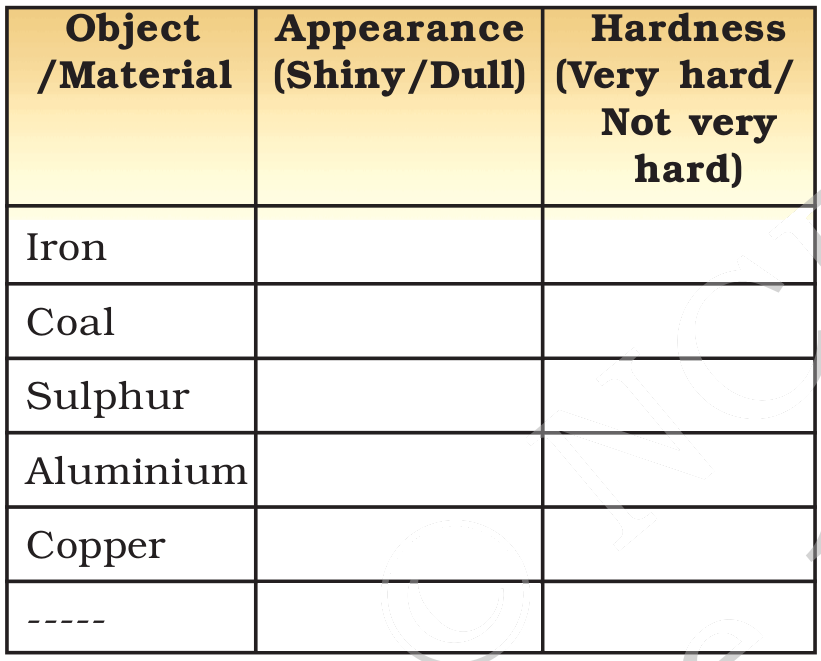
Can you name the materials which are metals? The rest of the materials in Table 4.1 are non-metals. Metals can be distinguished from non-metals on the basis of their physical and chemical properties. Recall that lustre and hardness are physical properties.
4.1 Physical Properties of Metals and Non-metals
Have you ever seen a blacksmith beating an iron piece or an article made up of iron, like a spade, a shovel, an axe? Do you find a change in the shape of these articles on beating? Would you expect a similar change if we try to beat a piece of coal ?
Let us find out.
Activity 4.1
Take a small iron nail, a coal piece, a piece of thick aluminium wire and a pencil lead. Beat the iron nail with a hammer (Fig. 4.1). (But take care that you don’t hurt yourself in the process.) Try to hit hard. Hit hard the aluminium wire also. Then repeat the same kind of treatment on the coal piece and pencil lead. Record your observations in Table 4.2.
Fig. 4.1 : Beating an iron nail with hammer

Table 4.2 : Malleability of Materials
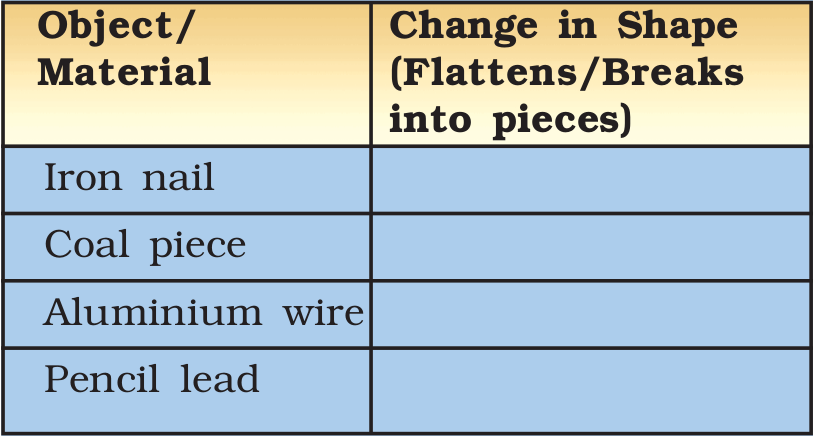
You saw that the shape of the iron nail and the aluminium wire changed on beating. If they were beaten harder these could be changed into sheets. You might be familiar with silver foil used for decorating sweets. You must also be familiar with the aluminium foil used for wrapping food. The property of metals by which they can be beaten into thin sheets is called malleability. This is a characteristic property of metals. As you must have noticed, materials like coal and pencil lead do not show this property. Can we call these metals?
Can you hold a hot metallic pan which is without a plastic or a wooden handle and not get hurt? Perhaps not! Why? Try to list some other experiences in which a wooden or plastic handle protects you from being hurt while handling hot things. On the basis of these experiences what can you say about the conduction of heat by wood and plastic?
You must have seen an electrician using his screw driver. What kind of handle does it have? Why?
Let us find out.
Activity 4.2
Recall how to make an electric circuit to test whether electricity can pass through an object or not (Fig. 4.2). You might have performed the activity with various objects in Class VI. Now, repeat the activity with the materials mentioned in Table 4.3. Observe and group these materials into good conductors and poor conductors.
Fig. 4.2 : Electric tester
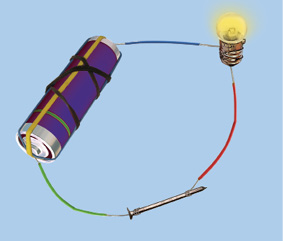
Table 4.3 : Electrical Conductivity of Materials
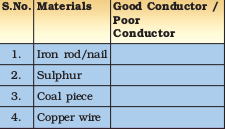
You observe that iron rod, nail and copper wire are good conductors while rolled sulphur piece and coal piece are poor conductors.
 Oh! The meaning of recalling our experiences and then of this activity was to show that metals are good conductors of heat and electricity. We learnt this in Class VI.
Oh! The meaning of recalling our experiences and then of this activity was to show that metals are good conductors of heat and electricity. We learnt this in Class VI.
Where do you find the use of aluminium and copper wires? Have you seen wires of coal? Definitely not!
The property of metal by which it can be drawn into wires is called ductility.
Have you ever noticed the difference in sound on dropping an iron sheet/plate, a metal coin, and a piece of coal on the floor? If not, you can try it now.
Do you note any difference in the sound produced?
Have you seen wooden bells in temples? Can you give a reason?
The things made of metals produce a ringing sound when struck hard. Suppose you have two boxes similar in appearance, one made of wood and the other of metal. Can you tell which box is made of metal by striking both the boxes?
Since metals produce ringing sounds, they are said to be sonorous. The materials other than metals are not sonorous.
After performing the above activities, we can say that some materials are hard, lustrous, malleable, ductile, sonorous and good conductors of heat and electricity. The materials which generally possess these properties are called metals. The examples of metals are iron, copper, aluminium, calcium, magnesium, etc. In contrast, materials like coal and sulphur are soft and dull in appearance. They break down into a powdery mass on tapping with a hammer. They are not sonorous and are poor conductors of heat and electricity. These materials are called non-metals. The examples of non-metals are sulphur, carbon, oxygen, phosphorus, etc.
Metals like sodium and potassium are soft and can be cut with a knife. Mercury is the only metal which is found in liquid state at room temperature. These are exceptions.
4.2 Chemical Properties of Metals and Non-metals
Reaction with Oxygen
You are familiar with the phenomenon of rusting of iron. Recall the reaction by which rust is formed. You had also performed in Class VII an activity of burning a magnesium ribbon in air. You had learnt that in both the processes oxide formation takes place. Complete the following reactions of iron and magnesium with oxygen.
Iron (Fe) + Oxygen (O2) + Water (H2O) → ?
Magnesium (Mg) + Oxygen (O2) → ?
Activity 4.3
Let us check the nature of rust formed as a result of the reaction between iron, oxygen and water. Collect a spoonful of rust and dissolve it in a very little amount of water. You will find that the rust remains suspended in water. Shake the suspension well. Test the solution with red and blue litmus papers (Fig. 4.3). What do you observe? Is the solution acidic or basic?
Fig. 4.3 : Testing the nature of rust

Does copper also get rusted? I have seen a greenish deposit on the surface ofcopper vessels.
When a copper vessel is exposed to moist air for long, it acquires a dull green coating. The green material is a mixture of copper hydroxide (Cu(OH) 2 ) and copper carbonate (CuCO 3 ). The following is the reaction 2Cu+H 2 O+CO 2 +O 2 →Cu (OH) 2 + CuCO 3
Now recall the activity of burning magnesium ribbon. The ash obtained on burning magnesium ribbon is dissolved in water and tested for its acidic/basic nature.
Is the solution acidic or basic? How do you ascertain this?
You must have observed that the red litmus turns blue. So, oxide of magnesium is also basic in nature. In general, metallic oxides are basic in nature.
Let us now observe the reaction of non-metals with oxygen.
Activity 4.4
(To be demonstrated by the teacher in the class)
Take a small amount of powdered sulphur in a deflagrating spoon and heat it. If deflagrating spoon is not available, you may take a metallic cap of any bottle and wrap a metallic wire around it and give it the shape shown in Fig. 4.4 (a).
As soon as sulphur starts burning, introduce the spoon into a gas jar/glass tumbler [Fig. 4.4 (a)]. Cover the tumbler with a lid to ensure that the gas produced does not escape. Remove the spoon after some time.
Add a small quantity of water into the tumbler and quickly replace the lid. Shake the tumbler well. Check the solution with red and blue litmus papers [Fig. 4.4 (b)].


Improvised deflagrating spoon


Table 4.4 : Metals and Non-metals in Acids and Bases
The name of the product formed in the reaction of sulphur and oxygen is sulphur dioxide gas. When sulphur dioxide is dissolved in water sulphurous acid is formed. The reaction can be given as follows:
Sulphur dioxide (SO2) + Water (H2O) → Sulphurous acid (H2SO3)
The sulphurous acid turns blue litmus paper red. Generally, oxides of non-metals are acidic in nature.
Recall the name of some of the laboratory acids and bases you have read in Class VII. Note down their names in Table 4.4. Identify the metal or non-metal present in them which forms oxides with oxygen.
Reaction with Water
Let us see how metals and non-metals react with water.
Activity 4.5
(To be demonstrated by the teacher. During demonstration special care should be taken that the size of the sodium metal piece is roughly the size of a wheat grain. It should be held with a pair of tongs.)
Take a 250 mL beaker/glass tumbler. Fill half of it with water. Now carefully cut a small piece of sodium metal. Dry it using filter paper and wrap it in a small piece of cotton. Put the sodium piece wrapped in cotton into the beaker. Observe carefully.(During observation keep away from the beaker). When reaction stops touch the beaker. What do you feel? Has the beaker become hot? Test the solution with red and blue litmus papers. Is the solution acidic or basic?
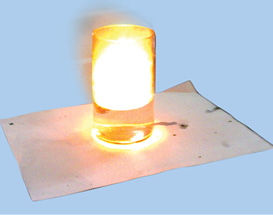
Fig. 4.5 : Reaction of sodium with water
You observed that sodium reacts vigorously with water. Some other metals do not do so. For example, iron reacts with water slowly.
Generally, non-metals do not react with water though they may be very reactive in air. Such non-metals are stored in water. For example, phosphorus is a very reactive non-metal. It catches fire if exposed to air. To prevent the contact of phosphorus with atmospheric oxygen, it is stored in water.
Reactions with Acids
Let us see how metals and non-metals behave with acids.
Activity 4.6
(Warning : Keep the mouth of the test tube away from your face. Use test tube holder to hold the test tube.)
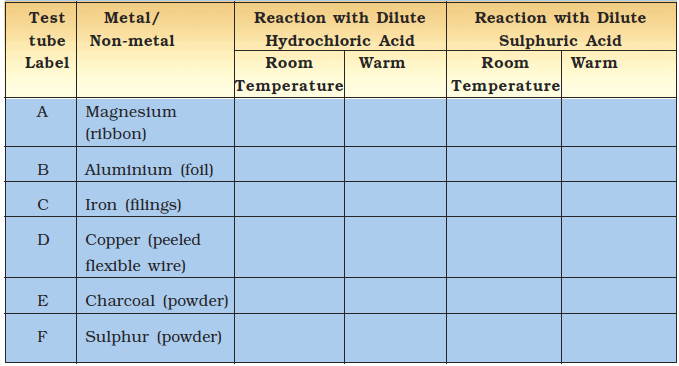
Take samples of metals and non-metals listed in Table 4.5 in separate test tubes and label them as A, B, C, D, E, and F. With the help of a dropper add 5 mL of dilute hydrochloric acid to each test tube one by one. Observe the reactions carefully. If no reaction occurs in the cold solution, warm the test tube gently. Bring a burning matchstick near the mouth of each test tube.
Repeat the same activity using dilute sulphuric acid instead of the dilute hydrochloric acid. Record your observations in Table 4.5.
Table 4.5 : Reaction of Metals and Non-metals with Acids
Is there a difference in the way metals and non-metals react with acids? What could the ‘pop’ sound in some cases be due to when a burning match stick is brought near the mouth of the test tubes?
You must have found that non-metals generally do not react with acids but metals react with acids and produce hydrogen gas that burns with a ‘pop’ sound. You must have noticed that copper does not react with dilute hydrochloric acid even on heating but it reacts with sulphuric acid.
Reactions with Bases
Activity 4.7
(To be demonstrated by the teacher. During the preparation of sodium hydroxide solution, care should be taken that pellets of sodium hydroxide are handled with a plastic spatula).
Prepare a fresh solution of sodium hydroxide in a test tube by dissolving 3-4 pellets of it in 5 mL of water. Drop a piece of aluminium foil into it. Bring a burning match stick near the mouth of the test tube. Observe carefully.
What does the ‘pop’ sound indicate? As before, the ‘pop’ sound indicates the presence of hydrogen gas.
Metals react with sodium hydroxide to produce hydrogen gas.
Reactions of non-metals with bases are complex.
Displacement Reactions
Recall the activity of the reaction between copper sulphate and iron that you performed in Class VII. Let us observe some more reactions of that kind.
Activity 4.8
Take five 100 mL beakers and label them A, B, C, D and E. Take about 50 mL of water in each beaker. Dissolve in each beaker a teaspoonful of each substance as indicated in Fig. 4.6 (a).
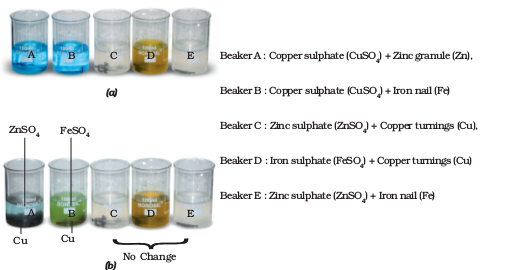
What changes do you observe in the various beakers? You have read that one metal displaces another metal from its compound in aqueous solution. In beaker ‘A’ zinc (Zn) replaces copper (Cu) from copper sulphate (CuSO4). That is why the blue colour of copper sulphate disappears and a powdery red mass of copper is deposited at the bottom of the beaker. The reaction can be represented as follows:
Copper Sulphate (CuSO4) + Zinc (Zn)
(Blue)
→ Zinc Sulphate (ZnSO4) + Copper (Cu)
(Colourless) (Red)
You can write down the reaction taking place in beaker ‘B’ in a similar manner.

I have understood the reactions taking place in beakers ‘A’ and ‘B’. But I am still confused why there is no change in beakers ‘C’, ‘D’ and ‘E’?
There could have been displacement of zinc by copper in beaker ‘C’ and by iron in beaker ‘E’. Similarly iron could be displaced by copper in beaker ‘D’.
Since we do not see any change in beaker C, we can infer that copper is not able to replace zinc from zinc sulphate. But why? When zinc can replace copper in beaker ‘A’ why cannot copper replace zinc in beaker ‘C’? Remember that science is not arbitrary. It follows definite rules based on facts. And the rule here is that zinc is more reactive than copper and iron. A more reactive metal can replace a less reactive metal, but a less reactive one cannot replace a more reactive metal. Now you can understand why there are no displacement reactions in beakers D and E also. Can you guess the sequence of metals from more reactive to less reactive among zinc, iron and copper?
4.3 Uses of Metals and Non-metals
You should be able to guess why metals are used in making machinery, automobiles, aeroplanes, trains, satellites, industrial gadgets, cooking utensils, water boilers, etc. You are also familiar with the uses of some non-metals. Here are some interesting ones. We are sure that you will guess them right:
Non-metal is essential for our life which all living beings inhale during breathing,
Non-metals used in fertilisers to enhance the growth of plants,
Non-metal used in water purification process,
Non-metal used in the purple coloured solution which is applied on wounds as an antiseptic,
Non-metals used in crackers.
You may add some more uses of metals and non-metals from your experiences.

I heard that magnesium is found in plants. In what form is it found in them?
Materials : Metals and Non-Metals

The doctor reported iron deficiency in my body. Where is iron in my body?
In Class VII, you have learnt that in a chemical reaction, new substances are formed. These substances are different from those which underwent the reaction. Now, if a substance cannot be broken down further by chemical reactions, by cooling, heating, or by electrolysis, it is called ‘element’. Sulphur is an element. So is iron. Carbon, too, is an element. The smallest unit of an element is atom. A sample of an element contains only one kind of atom. The atom of an element remains unaffected by physical changes in the element. For example, an atom of liquid sulphur would be exactly the same as the atom of solid or vapour sulphur.
Although we have an infinite variety of substances in the universe, the number of elements forming these substances is limited. There are no more than 94 naturally occurring elements. An important classification of elements is in terms of metals and non-metals. Most of the elements are metals. The remaining are either non-metals or metalloids. Metalloids possess character of both metals and non-metals.
Keywords
Atom
Conductor
displacement reaction
ductility
Elements
Hardness
Malleability
metals
Metalloids
Non-metals
Sonorous
What you have learnt
- Metals are lustrous whereas non-metals have no lustre.
- Generally, metals are malleable and ductile. Non-metals do not have these properties.
- Generally, metals are good conductors of heat and electricity but non-metals are poor conductors.
- On burning, metals react with oxygen to produce metal oxides which are basic in nature. Non-metals react with oxygen to produce non- metallic oxides which are acidic in nature.
- Some metals react with water to produce metal hydroxides and hydrogen gas. Generally, non-metals do not react with water.
- Metals react with acids and produce metal salts and hydrogen gas. Generally, non-metals do not react with acids.
- Some metals react with bases to produce hydrogen gas.
- More reactive metals displace less reactive metals from their compounds in aqueous solutions.
- Metals and non-metals are used widely in every day life.
Exercises
1. Which of the following can be bTeaten into thin sheets?
(a) Zinc (b) Phosphorus (c) Sulphur (d) Oxygen
2. Which of the following statements is correct?
(a) All metals are ductile.
(b) All non-metals are ductile.
(c) Generally, metals are ductile.
(d) Some non-metals are ductile.