Table of Contents

Chapter 14
Natural Resources
Our planet, Earth is the only one on which life, as we know it, exists. Life on Earth is dependent on many factors. Most life-forms we know need an ambient temperature, water, and food. The resources available on the Earth and the energy from the Sun are necessary to meet the basic requirements of all life-forms on the Earth.
What are these resources on the Earth?
These are the land, the water and the air. The outer crust of the Earth is called the lithosphere. Water covers 75% of the Earth’s surface. It is also found underground. These comprise the hydrosphere. The air that covers the whole of the Earth like a blanket, is called the atmosphere. Living things are found where these three exist. This life-supporting zone of the Earth where the atmosphere, the hydrosphere and the lithosphere interact and make life possible, is known as the biosphere.
Living things constitute the biotic component of the biosphere. The air, the water and the soil form the non-living or abiotic component of the biosphere. Let us study these abiotic components in detail in order to understand their role in sustaining life on Earth.
14.1 The Breath of Life: Air
We have already talked about the composition of air in the first chapter. It is a mixture of many gases like nitrogen, oxygen, carbon dioxide and water vapour. It is interesting to note that even the composition of air is the result of life on Earth. In planets such as Venus and Mars, where no life is known to exist, the major component of the atmosphere is found to be carbon dioxide. In fact, carbon dioxide constitutes up to 95-97% of the atmosphere on Venus and Mars.
Eukaryotic cells and many prokaryotic cells, discussed in Chapter 5, need oxygen to break down glucose molecules and get energy for their activities. This results in the production of carbon dioxide. Another process which results in the consumption of oxygen and the concomitant production of carbon dioxide is combustion. This includes not just human activities, which burn fuels to get energy, but also forest fires.
Despite this, the percentage of carbon dioxide in our atmosphere is a mere fraction of a percent because carbon dioxide is ‘fixed’ in two ways: (i) Green plants convert carbon dioxide into glucose in the presence of Sunlight and (ii) many marine animals use carbonates dissolved in sea-water to make their shells.
14.1.1 The role of the atmosphere in climate control
We have talked of the atmosphere covering the Earth, like a blanket. We know that air is a bad conductor of heat. The atmosphere keeps the average temperature of the Earth fairly steady during the day and even during the course of the whole year. The atmosphere prevents the sudden increase in temperature during the daylight hours. And during the night, it slows down the escape of heat into outer space. Think of the moon, which is about the same distance from the Sun that the Earth is. Despite that, on the surface of the moon, with no atmosphere, the temperature ranges from –190° C to 110° C.
Activity 14.1
• Measure the temperature of the following :
Take (i) a beaker full of water, (ii) a beaker full of soil/sand and (iii) a closed bottle containing a thermometer. Keep them in bright Sunlight for three hours. Now measure the temperature of all 3 vessels. Also, take the temperature reading in shade at the same time.
Now answer
1. Is the temperature reading more in activity (i) or (ii)?
2. Based on the above finding, which would become hot faster – the land or the sea?
3. Is the thermometer reading of the temperature of air (in shade) the same as the temperature of sand or water? What do you think is the reason for this? And why does the temperature have to be measured in the shade?
4. Is the temperature of air in the closed glass vessel/bottle the same as the temperature taken in open air? (i) What do you think is the reason for this? (ii) Do we ever come across this phenomenon in daily life?
As we have seen above, sand and water do not heat up at the same rate. What do you think will be their rates of cooling? Can we think of an experiment to test the prediction?
14.1.2 The movement of air: winds
We have all felt the relief brought by cool evening breezes after a hot day. And sometimes, we are lucky enough to get rains after some days of really hot weather. What causes the movement of air, and what decides whether this movement will be in the form of a gentle breeze, a strong wind or a terrible storm? What brings us the welcome rains?
All these phenomena are the result of changes that take place in our atmosphere due to the heating of air and the formation of water vapour. Water vapour is formed due to the heating of water bodies and the activities of living organisms. The atmosphere can be heated from below by the radiation that is reflected back or re-radiated by the land or water bodies. On being heated, convection currents are set up in the air. In order to gain some understanding of the nature of convection currents, let us perform the following activity:
Activity 14.2
• Place a candle in a beaker or wide-mouthed bottle and light it. Light an incense stick and take it to the mouth of the above bottle (Figure 14.1).
• Which way does the smoke flow when the incense stick is kept near the edge of the mouth?
• Which way does the smoke flow when the incense stick is kept a little above the candle?
• Which way does the smoke flow when the incense stick is kept in other regions?
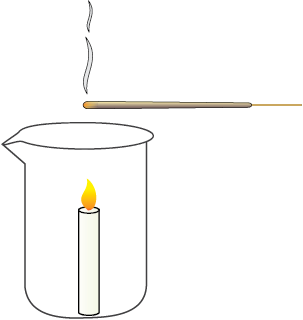
Fig. 14.1: Air currents being caused by the uneven heating of air.
The patterns revealed by the smoke show us the directions in which hot and cold air move. In a similar manner, when air is heated by radiation from the heated land or water, it rises. But since land gets heated faster than water, the air over land would also be heated faster than the air over water bodies.
So, if we look at the situation in coastal regions during the day, the air above the land gets heated faster and starts rising. As this air rises, a region of low pressure is created and air over the sea moves into this area of low pressure. The movement of air from one region to the other creates winds. During the day, the direction of the wind would be from the sea to the land.
At night, both land and sea start to cool. Since water cools down slower than the land, the air above water would be warmer than the air above land.
On the basis of the above discussion, what can you say about:
1. the appearance of areas of low and high pressure in coastal areas at night?
2. the direction in which air would flow at night in coastal areas?
Similarly, all the movements of air resulting in diverse atmospheric phenomena are caused by the uneven heating of the atmosphere in different regions of the Earth. But various other factors also influence these winds – the rotation of the Earth and the presence of mountain ranges in the paths of the wind are a couple of these factors. We will not go into these factors in detail in this chapter, but think about this: how do the presence of the Himalayas change the flow of a wind blowing from Allahabad towards the north?
14.1.3 Rain
Let us go back now to the question of how clouds are formed and bring us rain. We could start by doing a simple experiment which demonstrates some of the factors influencing these climatic changes.
Activity 14.3
• Take an empty bottle of the sort in which bottled water is sold. Pour about 5-10 mL of water into it and close the bottle tightly. Shake it well or leave it out in the Sun for ten minutes. This causes the air in the bottle to be saturated with water vapour.
• Now, take a lighted incense stick. Open the cap of the bottle and allow some of the smoke from the incense stick to enter the bottle. Quickly close the bottle once more. Make sure that the cap is fitting tightly. Press the bottle hard between your hands and crush it as much as possible. Wait for a few seconds and release the bottle. Again press the bottle as hard as you can.
Now answer
1. When did you observe that the air inside seemed to become ‘foggy’?
2. When does this fog disappear?
3. When is the pressure inside the bottle higher?
4. Is the ‘fog’ observed when the pressure in the bottle is high or when it is low?
5. What is the need for smoke particles inside the bottle for this experiment?
6. What might happen if you do the experiment without the smoke from the incense stick? Now try it and check if the prediction was correct. What might be happening in the above experiment in the absence of smoke particles?
The above experiment replicates, on a very small scale, what happens when air with a very high content of water vapour goes from a region of high pressure to a region of low pressure or vice versa.
When water bodies are heated during the day, a large amount of water evaporates and goes into the air. Some amount of water vapour also get into the atmosphere because of various biological activities. This air also gets heated. The hot air rises up carrying the water vapour with it. As the air rises, it expands and cools. This cooling causes the water vapour in the air to condense in the form of tiny droplets. This condensation of water is facilitated if some particles could act as the ‘nucleus’ for these drops to form around. Normally dust and other suspended particles in the air perform this function.
Once the water droplets are formed, they grow bigger by the ‘condensation’ of these water droplets. When the drops have grown big and heavy, they fall down in the form of rain. Sometimes, when the temperature of air is low enough, precipitation may occur in the form of snow, sleet or hail.
Rainfall patterns are decided by the prevailing wind patterns. In large parts of India, rains are mostly brought by the south-west or north-east monsoons. We have also heard weather reports that say ‘depressions’ in the Bay of Bengal have caused rains in some areas (Figure 14.2).
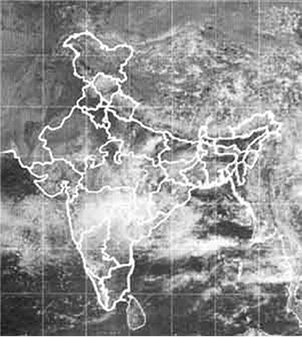
Fig. 14.2: Satellite picture showing clouds over India.
Activity 14.4
• Collect information from newspapers or weather reports on television about rainfall patterns across the country. Also find out how to construct a rain-gauge and make one. What precautions are necessary in order to get reliable data from this rain-gauge? Now answer the following questions :
• In which month did your city/town/ village get the maximum rainfall?
• In which month did your state/union territory get the maximum rainfall?
• Is rain always accompanied by thunder and lightning? If not, in which season do you get more of thunder and lightning with the rain?
Activity 14.5
• Find out more about monsoons and cyclones from the library. Try and find out the rainfall pattern of any other country. Is the monsoon responsible for rains the world over?
14.1.4 Air pollution
We keep hearing of the increasing levels of oxides of nitrogen and sulphur in the news. People often bemoan the fact that the quality of air has gone down since their childhood. How is the quality of air affected and how does this change in quality affect us and other life forms?
The fossil fuels like coal and petroleum contain small amounts of nitrogen and sulphur. When these fuels are burnt, nitrogen and sulphur too are burnt and this produces different oxides of nitrogen and sulphur. Not only is the inhalation of these gases dangerous, they also dissolve in rain to give rise to acid rain. The combustion of fossil fuels also increases the amount of suspended particles in air. These suspended particles could be unburnt carbon particles or substances called hydrocarbons. Presence of high levels of all these pollutants cause visibility to be lowered, especially in cold weather when water also condenses out of air. This is known as smog and is a visible indication of air pollution. Studies have shown that regularly breathing air that contains any of these substances increases the incidence of allergies, cancer and heart diseases. An increase in the content of these harmful substances in air is called air pollution.
Fig. 14.3: Lichen
Activity 14.6
• Organisms called lichens are found to be very sensitive to the levels of contaminants like sulphur dioxide in the air. As discussed earlier in section 7.3.3, lichens can be commonly found growing on the barks of trees as a thin greenish-white crust. See if you can find lichen growing on the trees in your locality.
• Compare the lichen on trees near busy roads and trees some distance away.
• On the trees near roads, compare the incidence of lichen on the side facing the road and on the side away from the road.
What can you say about the levels of polluting substances near roads and away from roads on the basis of your findings above?
Questions
1. How is our atmosphere different from the atmospheres on Venus and Mars?
2. How does the atmosphere act as a blanket?
3. What causes winds?
4. How are clouds formed?
5. List any three human activities that you think would lead to air pollution.
14.2 Water: A Wonder Liquid
Water occupies a very large area of the Earth’s surface and is also found underground. Some amount of water exists in the form of water vapour in the atmosphere. Most of the water on Earth’s surface is found in seas and oceans and is saline. Fresh water is found frozen in the ice-caps at the two poles and on snow-covered mountains. The underground water and the water in rivers, lakes and ponds is also fresh. However, the availability of fresh water varies from place to place. Practically every summer, most places have to face a shortage of water. And in rural areas, where water supply systems have not been installed, people are forced to spend considerable amounts of time in fetching water from far-away sources.
Activity 14.7
• Many municipal corporations are trying water-harvesting techniques to improve the availability of water.
• Find out what these techniques are and how they would increase the water that is available for use.
But why is water so necessary? And do all organisms require water? All cellular processes take place in a water medium. All the reactions that take place within our body and within the cells occur between substances that are dissolved in water. Substances are also transported from one part of the body to the other in a dissolved form. Hence, organisms need to maintain the level of water within their bodies in order to stay alive. Terrestrial life-forms require fresh water for this because their bodies cannot tolerate or get rid of the high amounts of dissolved salts in saline water. Thus, water sources need to be easily accessible for animals and plants to survive on land.
Activity 14.8
• Select a small area (say, 1 m2) near a water-body, it may be a river, stream, lake or pond. Count the number of different animals and plants in this area. Also, check the number of individuals of each type or species.
• Compare this with the number of individuals (both animals and plants) found in an area of the same size in a dry, rocky region.
• Is the variety of plant and animal life the same in both these areas?
Activity 14.9
• Select and mark out a small area (about 1 m2) in some unused land in or near your school.
• As in the above activity, count the number of different animals and plants in this area and the number of individuals of each species.
• Remember to do this in the same place twice in a year, once during summer or the dry season and once after it has rained.
Now answer
1. Were the numbers similar both times?
2. In which season did you find more variety of plants and animals?
3. In which season did you find more number of individuals of each variety?
After compiling the results of the above two activities, think if there is any relationship between the amount of available water and the number and variety of plants and animals that can live in a given area. If there is a relationship, where do you think you would find a greater variety and abundance of life – in a region that receives 5 cm of rainfall in a year or a region that receives 200 cm of rainfall in a year? Find the map showing rainfall patterns in the atlas and predict which States in India would have the maximum biodiversity and which would have the least. Can we think of any way of checking whether the prediction is correct?
The availability of water decides not only the number of individuals of each species that are able to survive in a particular area, but it also decides the diversity of life there. Of course, the availability of water is not the only factor that decides the sustainability of life in a region. Other factors like the temperature and nature of soil also matter. But water is one of the major resources which determine life on land.
14.2.1 Water pollution
Water dissolves the fertilisers and pesticides that we use on our farms. So some percentage of these substances are washed into the water bodies. Sewage from our towns and cities and the waste from factories are also dumped into rivers or lakes. Specific industries also use water for cooling in various operations and later return this hot water to water-bodies. Another manner in which the temperature of the water in rivers can be affected is when water is released from dams. The water inside the deep reservoir would be colder than the water at the surface which gets heated by the Sun.
All this can affect the life-forms that are found in these water bodies in various ways. It can encourage the growth of some life-forms and harm some other life-forms. This affects the balance between various organisms which had been established in that system. So we use the term water-pollution to cover the following effects:
1. The addition of undesirable substances to water-bodies. These substances could be the fertilisers and pesticides used in farming or they could be poisonous substances, like mercury salts which are used by paper-industries. These could also be disease-causing organisms, like the bacteria which cause cholera.
2. The removal of desirable substances from water-bodies. Dissolved oxygen is used by the animals and plants that live in water. Any change that reduces the amount of this dissolved oxygen would adversely affect these aquatic organisms. Other nutrients could also be depleted from the water bodies.
3. A change in temperature. Aquatic organisms are used to a certain range of temperature in the water-body where they live, and a sudden marked change in this temperature would be dangerous for them or affect their breeding. The eggs and larvae of various animals are particularly susceptible to temperature changes.
Questions
1. Why do organisms need water?
2. What is the major source of fresh water in the city/town/village where you live?
3. Do you know of any activity which may be polluting this water source?
14.3 Mineral Riches in the Soil
Soil is an important resource that decides the diversity of life in an area. But what is the soil and how is it formed? The outermost layer of our Earth is called the crust and the minerals found in this layer supply a variety of nutrients to life-forms. But these minerals will not be available to the organisms if the minerals are bound up in huge rocks. Over long periods of time, thousands and millions of years, the rocks at or near the surface of the Earth are broken down by various physical, chemical and some biological processes. The end product of this breaking down is the fine particles of soil. But what are the factors or processes that make soil?
• The Sun: The Sun heats up rocks during the day so that they expand. At night, these rocks cool down and contract. Since all parts of the rock do not expand and contract at the same rate, this results in the formation of cracks and ultimately the huge rocks break up into smaller pieces.
• Water: Water helps in the formation of soil in two ways. One, water could get into the cracks in the rocks formed due to uneven heating by the Sun. If this water later freezes, it would cause the cracks to widen. Can you think why this should be so? Two, flowing water wears away even hard rock over long periods of time. Fast flowing water often carries big and small particles of rock downstream. These rocks rub against other rocks and the resultant abrasion causes the rocks to wear down into smaller and smaller particles. The water then takes these particles along with it and deposits it further down its path. Soil is thus found in places far away from its parent-rock.
• Wind: In a process similar to the way in which water rubs against rocks and wears them down, strong winds also erode rocks down. The wind also carries sand from one place to the other like water does.
• Living organisms also influence the formation of soil. The lichen that we read about earlier, also grows on the surface of rocks. While growing, they release certain substances that cause the rock surface to powder down and form a thin layer of soil. Other small plants like moss, are able to grow on this surface now and they cause the rock to break up further. The roots of big trees sometimes go into cracks in the rocks and as the roots grow bigger, the crack is forced bigger.
Activity 14.10
• Take some soil and put it into a beaker containing water. The water should be at least five times the amount of soil taken. Stir the soil and water vigorously and allow the soil to settle down. Observe after some time.
• Is the soil at the bottom of the beaker homogenous or have layers formed?
• If layers have formed, how is one layer different from another?
• Is there anything floating on the surface of the water?
• Do you think some substances would have dissolved in the water? How would you check?
As you have seen, soil is a mixture. It contains small particles of rock (of different sizes). It also contains bits of decayed living organisms which is called humus. In addition, soil also contains various forms of microscopic life. The type of soil is decided by the average size of particles found in it and the quality of the soil is decided by the amount of humus and the microscopic organisms found in it. Humus is a major factor in deciding the soil structure because it causes the soil to become more porous and allows water and air to penetrate deep underground. The mineral nutrients that are found in a particular soil depends on the rocks it was formed from. The nutrient content of a soil, the amount of humus present in it and the depth of the soil are some of the factors that decide which plants will thrive on that soil. Thus, the topmost layer of the soil that contains humus and living organisms in addition to the soil particles is called the topsoil. The quality of the topsoil is an important factor that decides biodiversity in that area.
Modern farming practices involve the use of large amounts of fertilizers and pesticides. Use of these substances over long periods of time can destroy the soil structure by killing the soil micro-organisms that recycle nutrients in the soil. It also kills the Earthworms which are instrumental in making the rich humus. Fertile soil can quickly be turned barren if sustainable practices are not followed. Removal of useful components from the soil and addition of other substances, which adversely affect the fertility of the soil and kill the diversity of organisms that live in it, is called soil pollution.
The soil that we see today in one place has been created over a very long period of time. However, some of the factors that created the soil in the first place and brought the soil to that place may be responsible for the removal of the soil too. The fine particles of soil may be carried away by flowing water or wind. If all the soil gets washed away and the rocks underneath are exposed, we have lost a valuable resource because very little will grow on the rock.
Activity 14.11
• Take two identical trays and fill them with soil. Plant mustard or green gram or paddy in one of the trays and water both the trays regularly for a few days, till the first tray is covered by plant growth. Now, tilt both the trays and fix them in that position. Make sure that both the trays are tilted at the same angle. Pour equal amount of water gently on both trays such that the water flows out of the trays (Fig. 14.4).
• Study the amount of soil that is carried out of the trays. Is the amount the same in both the trays?
• Now pour equal amounts of water on both the trays from a height. Pour three or four times the amount that you poured earlier.
• Study the amount of soil that is carried out of the trays now. Is the amount the same in both the trays?
• Is the amount of soil that is carried out more or less or equal to the amount washed out earlier?
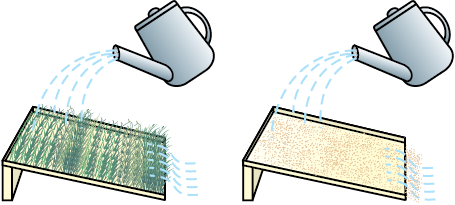
Fig. 14.4: Effect of flowing water on the top-soil
The roots of plants have an important role in preventing soil erosion. The large-scale deforestation that is happening all over the world not only destroys biodiversity, it also leads to soil erosion. Topsoil that is bare of vegetation, is likely to be removed very quickly. And this is accelerated in hilly or mountainous regions. This process of soil erosion is very difficult to reverse. Vegetative cover on the ground has a role to play in the percolation of water into the deeper layers too.
Questions
1. How is soil formed?
2. What is soil erosion?
3. What are the methods of preventing or reducing soil erosion?
14.4 Biogeochemical Cycles
A constant interaction between the biotic and abiotic components of the biosphere makes it a dynamic, but stable system. These interactions consist of a transfer of matter and energy between the different components of the biosphere. Let us look at some processes involved in the maintenance of the above balance.
14.4.1 The water-cycle
You have seen how the water evaporates from the water bodies and subsequent condensation of this water vapour leads to rain. But we don’t see the seas and oceans drying up. So, how is the water returning to these water bodies? The whole process in which water evaporates and falls on the land as rain and later flows back into the sea via rivers is known as the water-cycle. This cycle is not as straight-forward and simple as this statement seems to imply. All of the water that falls on the land does not immediately flow back into the sea. Some of it seeps into the soil and becomes part of the underground reservoir of fresh-water. Some of this underground water finds its way to the surface through springs. Or we bring it to the surface for our use through wells or tube-wells. Water is also used by terrestrial animals and plants for various life-processes (Fig. 14.5).
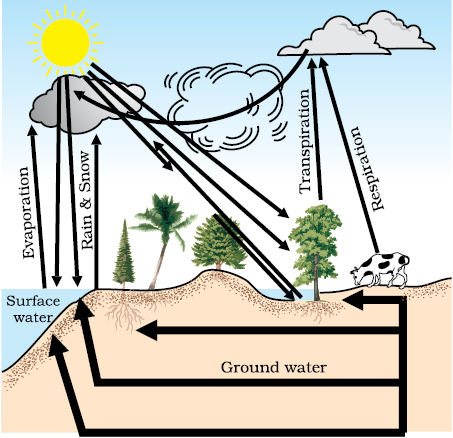
Fig. 14.5: Water-cycle in nature
Let us look at another aspect of what happens to water during the water-cycle. As you know, water is capable of dissolving a large number of substances. As water flows through or over rocks containing soluble minerals, some of them get dissolved in the water. Thus rivers carry many nutrients from the land to the sea, and these are used by the marine organisms.
14.4.2 The nitrogen-cycle
Nitrogen gas makes up 78% of our atmosphere and nitrogen is also a part of many molecules essential to life like proteins, nucleic acids (DNA and RNA) and some vitamins. Nitrogen is found in other biologically important compounds such as alkaloids and urea too. Nitrogen is thus an essential nutrient for all life-forms and life would be simple if all these life-forms could use the atmospheric nitrogen directly. However, other than a few forms of bacteria, life-forms are not able to convert the comparatively inert nitrogen molecule into forms like nitrates and nitrites which can be taken up and used to make the required molecules. These ‘nitrogen-fixing’ bacteria may be free-living or be associated with some species of dicot plants. Most commonly, the nitrogen-fixing bacteria are found in the roots of legumes (generally the plants which give us pulses) in special structures called root-nodules. Other than these bacteria, the only other manner in which the nitrogen molecule is converted to nitrates and nitrites is by a physical process. During lightning, the high temperatures and pressures created in the air convert nitrogen into oxides of nitrogen. These oxides dissolve in water to give nitric and nitrous acids and fall on land along with rain. These are then utilised by various life-forms.
What happens to the nitrogen once it is converted into forms that can be taken up and used to make nitrogen-containing molecules? Plants generally take up nitrates and nitrites and convert them into amino acids which are used to make proteins. Some other biochemical pathways are used to make the other complex compounds containing nitrogen. These proteins and other complex compounds are subsequently consumed by animals. Once the animal or the plant dies, other bacteria in the soil convert the various compounds of nitrogen back into nitrates and nitrites. A different type of bacteria converts the nitrates and nitrites into elemental nitrogen. Thus, there is a nitrogen-cycle in nature in which nitrogen passes from its elemental form in the atmosphere into simple molecules in the soil and water, which get converted to more complex molecules in living beings and back again to the simple nitrogen molecule in the atmosphere.
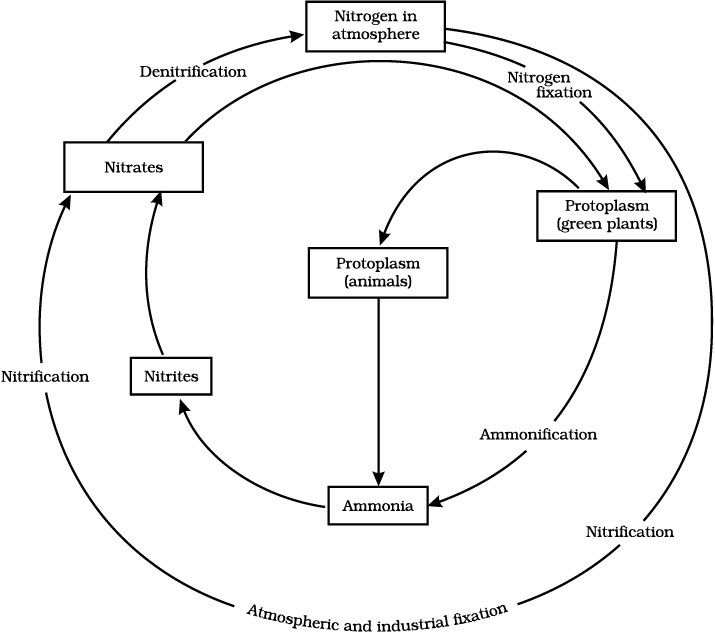
Fig.14.6: Nitrogen-cycle in nature
14.4.3 The carbon-cycle
Carbon is found in various forms on the Earth. It occurs in the elemental form as diamonds and graphite. In the combined state, it is found as carbon dioxide in the atmosphere, as carbonate and hydrogen- carbonate salts in various minerals, while all life-forms are based on carbon-containing molecules like proteins, carbohydrates, fats, nucleic acids and vitamins. The endoskeletons and exoskeletons of various animals are also formed from carbonate salts. Carbon is incorporated into life-forms through the basic process of photosynthesis which is performed in the presence of Sunlight by all life-forms that contain chlorophyll. This process converts carbon dioxide from the atmosphere or dissolved in water into glucose molecules. These glucose molecules are either converted into other substances or used to provide energy for the synthesis of other biologically important molecules (Fig. 14.7).
The utilisation of glucose to provide energy to living things involves the process of respiration in which oxygen may or may not be used to convert glucose back into carbon dioxide. This carbon dioxide then goes back into the atmosphere. Another process that adds to the carbon dioxide in the atmosphere is the process of combustion where fuels are burnt to provide energy for various needs like heating, cooking, transportation and industrial processes. In fact, the percentage of carbon dioxide in the atmosphere is said to have doubled since the industrial revolution when human beings started burning fossil fuels on a very large scale. Carbon, like water, is thus cycled repeatedly through different forms by the various physical and biological activities.
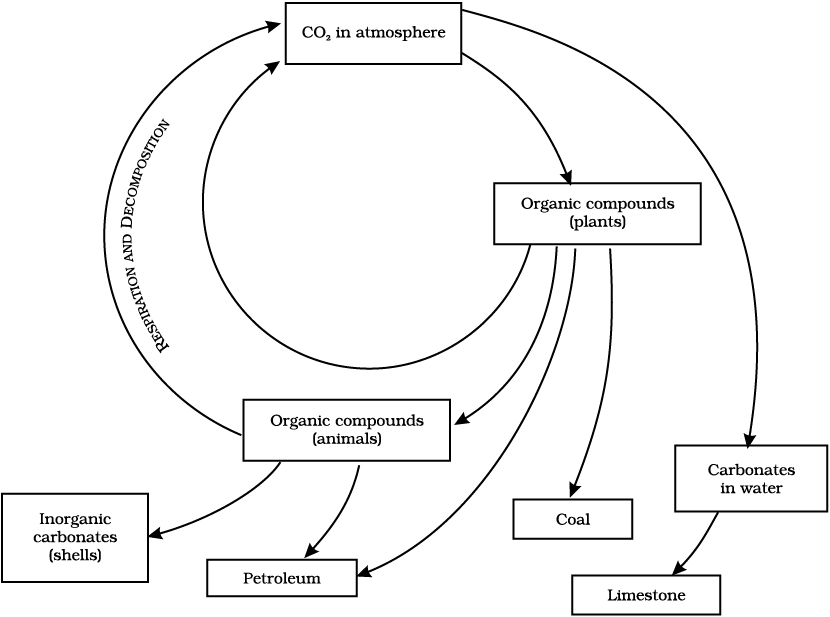
Fig. 14.7: Carbon-cycle in nature
14.4.3 (i) The greenhouse effect
Recall the reading taken by you under (iii) in Activity 14.1. Heat is trapped by glass, and hence the temperature inside a glass enclosure will be much higher than the surroundings. This phenomenon was used to create an enclosure where tropical plants could be kept warm during the winters in colder climates. Such enclosures are called greenhouses. Greenhouses have also lent their name to an atmospheric phenomenon. Some gases prevent the escape of heat from the Earth. An increase in the percentage of such gases in the atmosphere would cause the average temperatures to increase world-wide and this is called the greenhouse effect. Carbon dioxide is one of the greenhouse gases. An increase in the carbon dioxide content in the atmosphere would cause more heat to be retained by the atmosphere and lead to global warming.
Activity 14.12
• Find out what the consequences of global warming would be.
• Also, find out the names of some other greenhouse gases.
14.4.4 The oxygen-cycle
Oxygen is a very abundant element on our Earth. It is found in the elemental form in the atmosphere to the extent of 21%. It also occurs extensively in the combined form in the Earth’s crust as well as also in the air in the form of carbon dioxide. In the crust, it is found as the oxides of most metals and silicon, and also as carbonate, sulphate, nitrate and other minerals. It is also an essential component of most biological molecules like carbohydrates, proteins, nucleic acids and fats (or lipids).
But when we talk of the oxygen-cycle, we are mainly referring to the cycle that maintains the levels of oxygen in the atmosphere. Oxygen from the atmosphere is used up in three processes, namely combustion, respiration and in the formation of oxides of nitrogen. Oxygen is returned to the atmosphere in only one major process, that is, photosynthesis. And this forms the broad outline of the oxygen-cycle in nature (Fig. 14.8).
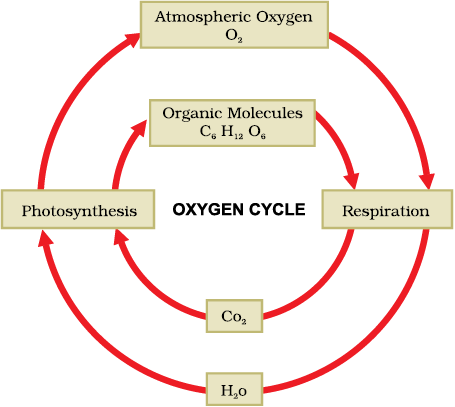
Fig. 14.8: Oxygen-cycle in nature
Though we usually think of oxygen as being necessary to life in the process of respiration, it might be of interest to you to learn that some forms of life, especially bacteria, are poisoned by elemental oxygen. In fact, even the process of nitrogen-fixing by bacteria does not take place in the presence of oxygen.
14.5 Ozone Layer
Elemental oxygen is normally found in the form of a diatomic molecule. However, in the upper reaches of the atmosphere, a molecule containing three atoms of oxygen is found. This would mean a formula of O3 and this is called ozone. Unlike the normal diatomic molecule of oxygen, ozone is poisonous and we are lucky that it is not stable nearer to the Earth’s surface. But it performs an essential function where it is found. It absorbs harmful radiations from the Sun. This prevents those harmful radiations from reaching the surface of the Earth where they may damage many forms of life.
Recently it was discovered that this ozone layer was getting depleted. Various man-made compounds like CFCs (carbon compounds having both fluorine and chlorine which are very stable and not degraded by any biological process) were found to persist in the atmosphere. Once they reached the ozone layer, they would react with the ozone molecules. This resulted in a reduction of the ozone layer and recently they have discovered a hole in the ozone layer above the Antartica. It is difficult to imagine the consequences for life on Earth if the ozone layer dwindles further, but many people think that it would be better not to take chances. These people advocate working towards stopping all further damage to the ozone layer.

oct 1980 oct 1985 oct 1990
Fig. 14.9: Satellite picture showing the hole (magenta colour) in the ozone layer over Antartica
Activity 14.13
• Find out which other molecules are thought to damage the ozone layer.
• Newspaper reports often talk about the hole in the ozone layer.
• Find out whether the size of this hole is changing and in what manner scientists think this would affect life on Earth (Fig. 14.9).
Questions
1. What are the different states in which water is found during the water cycle?
2. Name two biologically important compounds that contain both oxygen and nitrogen.
3. List any three human activities which would lead to an increase in the carbon dioxide content of air.
4. What is the greenhouse effect?
5. What are the two forms of oxygen found in the atmosphere?
What you have learnt
• Life on Earth depends on resources like soil, water and air, and energy from the Sun.
• Uneven heating of air over land and water-bodies causes winds.
• Evaporation of water from water-bodies and subsequent condensation give us rain.
• Rainfall patterns depend on the prevailing wind patterns in an area.
• Various nutrients are used again and again in a cyclic fashion. This leads to a certain balance between the various components of the biosphere.
• Pollution of air, water and soil affect the quality of life and harm the biodiversity.
• We need to conserve our natural resources and use them in a sustainable manner.
Exercises
1. Why is the atmosphere essential for life?
2. Why is water essential for life?
3. How are living organisms dependent on the soil? Are organisms that live in water totally independent of soil as a resource?
4. You have seen weather reports on television and in newspapers. How do you think we are able to predict the weather?
5. We know that many human activities lead to increasing levels of pollution of the air, water-bodies and soil. Do you think that isolating these activities to specific and limited areas would help in reducing pollution?
6. Write a note on how forests influence the quality of our air, soil and water resources.