Table of Contents

Unit 2
Structure of Atom
![1717.png]() Objectives
Objectives
After studying this unit you will be able to
• know about the discovery of electron, proton and neutron and their characteristics;
• describe Thomson, Rutherford and Bohr atomic models;
• understand the important features of the quantum mechanical model of atom;
• understand nature of electromagnetic radiation and Planck’s quantum theory;
• explain the photoelectric effect and describe features of atomic spectra;
• state the de Broglie relation and Heisenberg uncertainty principle;
• define an atomic orbital in terms of quantum numbers;
• state aufbau principle, Pauli exclusion principle and Hund’s rule of maximum multiplicity; and
• write the electronic configurations of atoms.
The rich diversity of chemical behaviour of different elements can be traced to the differences in the internal structure of atoms of these elements.
The existence of atoms has been proposed since the time of early Indian and Greek philosophers (400 B.C.) who were of the view that atoms are the fundamental building blocks of matter. According to them, the continued subdivisions of matter would ultimately yield atoms which would not be further divisible. The word ‘atom’ has been derived from the Greek word ‘a-tomio’ which means ‘uncut-able’ or ‘non-divisible’. These earlier ideas were mere speculations and there was no way to test them experimentally. These ideas remained dormant for a very long time and were revived again by scientists in the nineteenth century.
The atomic theory of matter was first proposed on a firm scientific basis by John Dalton, a British school teacher in 1808. His theory, called Dalton’s atomic theory, regarded the atom as the ultimate particle of matter (Unit 1). Dalton’s atomic theory was able to explain the law of conservation of mass, law of constant composition and law of multiple proportion very successfully. However, it failed to explain the results of many experiments, for example, it was known that substances like glass or ebonite when rubbed with silk or fur get electrically charged.
In this unit we start with the experimental observations made by scientists towards the end of nineteenth and beginning of twentieth century. These established that atoms are made of sub-atomic particles, i.e., electrons, protons and neutrons — a concept very different from that of Dalton.
2.1 Discovery of Sub-atomic Particles
An insight into the structure of atom was obtained from the experiments on electrical discharge through gases. Before we discuss these results we need to keep in mind a basic rule regarding the behaviour of charged particles : “Like charges repel each other and unlike charges attract each other”.
2.1.1 Discovery of Electron
In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the liberation and deposition of matter at the electrodes. He formulated certain laws which you will study in class XII. These results suggested the particulate nature of electricity.
In mid 1850s many scientists mainly Faraday began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes. It is depicted in Fig. 2.1. A cathode ray tube is made of glass containing two thin pieces of metal, called electrodes, sealed in it. The electrical discharge through the gases could be observed only at very low pressures and at very high voltages. The pressure of different gases could be adjusted by evacuation of the glass tubes. When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode (cathode) to the positive electrode (anode). These were called cathode rays or cathode ray particles. The flow of current from cathode to anode was further checked by making a hole in the anode and coating the tube behind anode with phosphorescent material zinc sulphide. When these rays, after passing through anode, strike the zinc sulphide coating, a bright spot is developed on the coating [Fig. 2.1(b)].
The results of these experiments are summarised below.
(i) The cathode rays start from cathode and move towards the anode.
(ii) These rays themselves are not visible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by them. Television picture tubes are cathode ray tubes and television pictures result due to fluorescence on the television screen coated with certain fluorescent or phosphorescent materials.
(iii) In the absence of electrical or magnetic field, these rays travel in straight lines (Fig. 2.2).
(iv) In the presence of electrical or magnetic field, the behaviour of cathode rays are similar to that expected from negatively charged particles, suggesting that the cathode rays consist of negatively charged particles, called electrons.
(v) The characteristics of cathode rays (electrons) do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.
Thus, we can conclude that electrons are basic constituent of all the atoms.
2.1.2 Charge to Mass Ratio of Electron
In 1897, British physicist J.J. Thomson measured the ratio of electrical charge (e) to the mass of electron (me ) by using cathode ray tube and applying electrical and magnetic field perpendicular to each other as well as to the path of electrons (Fig. 2.2). When only electric field is applied, the electrons deviate from their path and hit the cathode ray tube at point A (Fig. 2.2). Similarly when only magnetic field is applied, electron strikes the cathode ray tube at point C. By carefully balancing the electrical and magnetic field strength, it is possible to bring back the electron to the path which is followed in the absence of electric or magnetic field and they hit the screen at point B. Thomson argued that the amount of deviation of the particles from their path in the presence of electrical or magnetic field depends upon:
(i) the magnitude of the negative charge on the particle, greater the magnitude of the charge on the particle, greater is the interaction with the electric or magnetic field and thus greater is the deflection.
(ii) the mass of the particle — lighter the particle, greater the deflection.
(iii) the strength of the electrical or magnetic field — the deflection of electrons from its original path increases with the increase in the voltage across the electrodes, or the strength of the magnetic field.
By carrying out accurate measurements on the amount of deflections observed by the electrons on the electric field strength or magnetic field strength, Thomson was able to determine the value of e/me as:
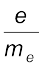 = 1.758820 × 1011 C kg–1 (2.1)
= 1.758820 × 1011 C kg–1 (2.1)
Where me is the mass of the electron in kg and e is the magnitude of the charge on the electron in coulomb (C). Since electrons are negatively charged, the charge on electron is –e.
2.1.3 Charge on the Electron
R.A. Millikan (1868-1953) devised a method known as oil drop experiment (1906-14), to determine the charge on the electrons. He found the charge on the electron to be
– 1.6 × 10–19 C. The present accepted value of electrical charge is – 1.602176 × 10–19 C. The mass of the electron (me) was determined by combining these results with Thomson’s value of e/me ratio.
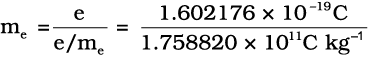
= 9.1094×10–31 kg (2.2)
2.1.4 Discovery of Protons and Neutrons
Electrical discharge carried out in the modified cathode ray tube led to the discovery of canal rays carrying positively charged particles. The characteristics of these positively charged particles are listed below.
(i) Unlike cathode rays, mass of positively charged particles depends upon the nature of gas present in the cathode ray tube. These are simply the positively charged gaseous ions.
(ii) The charge to mass ratio of the particles depends on the gas from which these originate.
(iii) Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
(iv) The behaviour of these particles in the magnetic or electrical field is opposite to that observed for electron or cathode rays.
The smallest and lightest positive ion was obtained from hydrogen and was called proton. This positively charged particle was characterised in 1919. Later, a need was felt for the presence of electrically neutral particle as one of the constituent of atom. These particles were discovered by Chadwick (1932) by bombarding a thin sheet of beryllium by α-particles. When electrically neutral particles having a mass slightly greater than that of protons were emitted. He named these particles as neutrons. The important properties of all these fundamental particles are given in Table 2.1.
2.2 Atomic Models
Observations obtained from the experiments mentioned in the previous sections have suggested that Dalton’s indivisible atom is composed of sub-atomic particles carrying positive and negative charges. The major problems before the scientists after the discovery of sub-atomic particles were:
• to account for the stability of atom,
• to compare the behaviour of elements in terms of both physical and chemical properties,
• to explain the formation of different kinds of molecules by the combination of different atoms and,
• to understand the origin and nature of the characteristics of electromagnetic radiation absorbed or emitted by atoms.
Different atomic models were proposed to explain the distributions of these charged particles in an atom. Although some of these models were not able to explain the stability of atoms, two of these models, one proposed by J.J. Thomson and the other proposed by Ernest Rutherford are discussed below.
2.2.1 Thomson Model of Atom
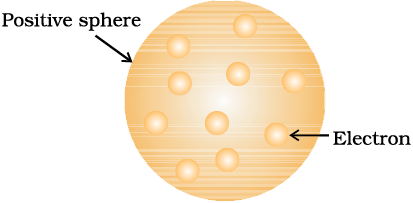
J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement (Fig. 2.4). Many different names are given to this model, for example, plum pudding, raisin pudding or watermelon. This model can be visualised as a pudding or watermelon of positive charge with plums or seeds (electrons) embedded into it. An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom. Although this model was able to explain the overall neutrality of the atom, but was not consistent with the results of later experiments. Thomson was awarded Nobel Prize for physics in 1906, for his theoretical and experimental investigations on the conduction of electricity by gases.
Millikan’s Oil Drop Method
In this method, oil droplets in the form of mist, produced by the atomiser, were allowed to enter through a tiny hole in the upper plate of electrical condenser. The downward motion of these droplets was viewed through the telescope, equipped with a micrometer eye piece. By measuring the rate of fall of these droplets, Millikan was able to measure the mass of oil droplets. The air inside the chamber was ionized by passing a beam of X-rays through it. The electrical charge on these oil droplets was acquired by collisions with gaseous ions. The fall of these charged oil droplets can be retarded, accelerated or made stationary depending upon the charge on the droplets and the polarity and strength of the voltage applied to the plate. By carefully measuring the effects of electrical field strength on the motion of oil droplets, Millikan concluded that the magnitude of electrical charge, q, on the droplets is always an integral multiple of the electrical charge, e, that is, q = n e, where n = 1, 2, 3... .
Fig.2.4 Thomson model of atom
In the later half of the nineteenth century different kinds of rays were discovered, besides those mentioned earlier.
Wilhalm Röentgen (1845-1923) in 1895 showed that when electrons strike a material in the cathode ray tubes,produce rays which can
cause fluorescence in the fluorescent materials placed outside the cathode ray tubes.Since Röentgen did not
know the nature of the radiation, he named them X-rays and the name is still carried on. Itwas noticed that X-rays are produced effectively when electrons strike the dense metal anode, called targets.These are not
deflected by the electric and magnetic fields and have a very high penetrating power through thematter and that
is the reason that these rays are used to study the interior of the objects. These rays are of veryshort wavelengths
(∼0.1 nm) and possess electro-magnetic character (Section 2.3.1).
Henri Becqueral (1852-1908) observed that there are certain elements which emit radiation on their own and named this phenomenon as radioactivity and the elements known as radioactive elements. This field was developed by Marie Curie, Piere Curie, Rutherford and Fredrick Soddy. It was observed that three kinds of rays i.e., α, β- and γ-rays are emitted. Rutherford found that α-rays consists of high energy particles carrying two units of positive charge and four unit of atomic mass. He concluded that α- particles are helium nuclei as when α- particles combined with two electrons yielded helium gas. β-rays are negatively charged particles similar to electrons. The γ-rays are high energy radiations like X-rays, are neutral in nature and do not consist of particles. As regards penetrating power, α-particles are the least, followed by β-rays (100 times that of α–particles) and γ-rays (1000 times of that α-particles).
2.2.2 Rutherford’s Nuclear Model of Atom
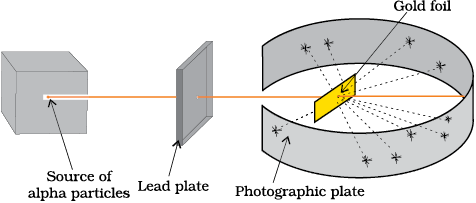
Rutherford and his students (Hans Geiger and Ernest Marsden) bombarded very thin gold foil with α–particles. Rutherford’s famous
α–particle scattering experiment is represented in Fig. 2.5. A stream of high energy α–particles from a radioactive source was directed at a thin foil (thickness ∼ 100 nm) of gold metal. The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever α–particles struck the screen, a tiny flash of light was produced at that point.
The results of scattering experiment were quite unexpected. According to Thomson model of atom, the mass of each gold atom in the foil should have been spread evenly over the entire atom, and α– particles had enough energy to pass directly through such a uniform distribution of mass. It was expected that the particles would slow down and change directions only by a small angles as they passed through the foil. It was observed that:
(i) most of the α–particles passed through the gold foil undeflected.
(ii) a small fraction of the α–particles was deflected by small angles.
(iii) a very few α–particles (∼1 in 20,000) bounced back, that is, were deflected by nearly 180°.
On the basis of the observations, Rutherford drew the following conclusions regarding the structure of atom:
(i) Most of the space in the atom is empty as most of the α–particles passed through the foil undeflected.
(ii) A few positively charged α–particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson had presumed. The positive charge has to be concentrated in a very small volume that repelled and deflected the positively charged α–particles.

A. Rutherford’s scattering experiment
Fig. 2.5 Schematic view of Rutherford’s scattering experiment. When a beam of alpha (α) particles is “shot” at a thin gold foil, most of them pass through without much effect. Some, however, are deflected.
B. Schematic molecular view of the gold foil
(iii) Calculations by Rutherford showed that the volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10–10 m, while that of nucleus is 10–15 m. One can appreciate this difference in size by realising that if a cricket ball represents a nucleus, then the radius of atom would be about 5 km.
On the basis of above observations and conclusions, Rutherford proposed the nuclear model of atom. According to this model:
(i) The positive charge and most of the mass of the atom was densely concentrated in extremely small region. This very small portion of the atom was called nucleus by Rutherford.
(ii) The nucleus is surrounded by electrons that move around the nucleus with a very high speed in circular paths called orbits. Thus, Rutherford’s model of atom resembles the solar system in which the nucleus plays the role of sun and the electrons that of revolving planets.
(iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
2.2.3 Atomic Number and Mass Number
The presence of positive charge on the nucleus is due to the protons in the nucleus. As established earlier, the charge on the proton is equal but opposite to that of electron. The number of protons present in the nucleus is equal to atomic number (Z ). For example, the number of protons in the hydrogen nucleus is 1, in sodium atom it is 11, therefore their atomic numbers are 1 and 11 respectively. In order to keep the electrical neutrality, the number of electrons in an atom is equal to the number of protons (atomic number, Z ). For example, number of electrons in hydrogen atom and sodium atom are 1 and 11 respectively.
Atomic number (Z) = number of protons in the nucleus of an atom
= number of electrons
in a nuetral atom (2.3)
While the positive charge of the nucleus is due to protons, the mass of the nucleus, due to protons and neutrons. As discussed earlier protons and neutrons present in the nucleus are collectively known as nucleons. The total number of nucleons is termed as mass number (A) of the atom.
mass number (A) = number of protons (Z)
+ number of neutrons (n) (2.4)
2.2.4 Isobars and Isotopes
The composition of any atom can be represented by using the normal element symbol (X) with super-script on the left hand side as the atomic mass number (A) and subscript (Z) on the left hand side as the atomic number (i.e., AZ X).
Isobars are the atoms with same mass number but different atomic number for example, 614C and 714N. On the other hand, atoms with identical atomic number but different atomic mass number are known as Isotopes. In other words (according to equation 2.4), it is evident that difference between the isotopes is due to the presence of different number of neutrons present in the nucleus. For example, considering of hydrogen atom again, 99.985% of hydrogen atoms contain only one proton. This isotope is called protium (11H). Rest of the percentage of hydrogen atom contains two other isotopes, the one containing 1 proton and 1 neutron is called deuterium (12D, 0.015%) and the other one possessing 1 proton and 2 neutrons is called tritium (13T ). The latter isotope is found in trace amounts on the earth. Other examples of commonly occuring isotopes are: carbon atoms containing 6, 7 and 8 neutrons besides 6 protons (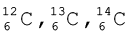 ); chlorine atoms containing 18 and 20 neutrons besides 17 protons (
); chlorine atoms containing 18 and 20 neutrons besides 17 protons ( ).
).
Lastly an important point to mention regarding isotopes is that chemical properties of atoms are controlled by the number of electrons, which are determined by the number of protons in the nucleus. Number of neutrons present in the nucleus have very little effect on the chemical properties of an element. Therefore, all the isotopes of a given element show same chemical behaviour.
Problem 2.1
Calculate the number of protons, neutrons and electrons in 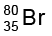 .
.
Solution
In this case, 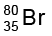 , Z = 35, A = 80, species is neutral
, Z = 35, A = 80, species is neutral
Number of protons = number of electrons = Z = 35
Number of neutrons = 80 – 35 = 45, (equation 2.4)
Problem 2.2
The number of electrons, protons and neutrons in a species are equal to 18, 16 and 16 respectively. Assign the proper symbol to the species.
Solution
The atomic number is equal to
number of protons = 16. The element is sulphur (S).
Atomic mass number = number of protons + number of neutrons
= 16 + 16 = 32
Species is not neutral as the number of protons is not equal to electrons. It is anion (negatively charged) with charge equal to excess electrons = 18 – 16 = 2. Symbol is 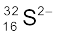 .
.
Note : Before using the notation AZ X, find out whether the species is a neutral atom, a cation or an anion. If it is a neutral atom, equation (2.3) is valid, i.e., number of protons = number of electrons = atomic number. If the species is an ion, determine whether the number of protons are larger (cation, positive ion) or smaller (anion, negative ion) than the number of electrons. Number of neutrons is always given by A–Z, whether the species is neutral or ion.
2.2.5 Drawbacks of Rutherford Model
As you have learnt above, Rutherford nuclear model of an atom is like a small scale solar system with the nucleus playing the role of the massive sun and the electrons being similar to the lighter planets. When classical mechanics* is applied to the solar system, it shows that the planets describe well-defined orbits around the sun. The gravitational force between the planets is given by the expression 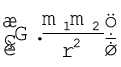 where m1 and m2 are the masses, r is the distance of separation of the masses and G is the gravitational constant. The theory can also calculate precisely the planetary orbits and these are in agreement with the experimental measurements.
where m1 and m2 are the masses, r is the distance of separation of the masses and G is the gravitational constant. The theory can also calculate precisely the planetary orbits and these are in agreement with the experimental measurements.
The similarity between the solar system and nuclear model suggests that electrons should move around the nucleus in well defined orbits. Further, the coulomb force (kq1q2/r2 where q1 and q2 are the charges, r is the distance of separation of the charges and k is the proportionality constant) between electron and the nucleus is mathematically similar to the gravitational force. However, when a body is moving in an orbit, it undergoes acceleration even if it is moving with a constant speed in an orbit because of changing direction. So an electron in the nuclear model describing planet like orbits is under acceleration. According to the electromagnetic theory of Maxwell, charged particles when accelerated should emit electromagnetic radiation (This feature does not exist for planets since they are uncharged). Therefore, an electron in an orbit will emit radiation, the energy carried by radiation comes from electronic motion. The orbit will thus continue to shrink. Calculations show that it should take an electron only 10–8 s to spiral into the nucleus. But this does not happen. Thus, the Rutherford model cannot explain the stability of an atom.
If the motion of an electron is described on the basis of the classical mechanics and electromagnetic theory, you may ask that since the motion of electrons in orbits is leading to the instability of the atom, then why not consider electrons as stationary around the nucleus. If the electrons were stationary, electrostatic attraction between the dense nucleus and the electrons would pull the electrons toward the nucleus to form a miniature version o Thomson’s model of atom.
Another serious drawback of the Rutherford model is that it says nothing about distribution of the electrons around the nucleus and the energies of these electrons.
2.3 Developments Leading to the Bohr’s Model of Atom
Historically, results observed from the studies of interactions of radiations with matter have provided immense information regarding the structure of atoms and molecules. Neils Bohr utilised these results to improve upon the model proposed by Rutherford. Two developments played a major role in the formulation of Bohr’s model of atom. These were:
(i) Dual character of the electromagnetic radiation which means that radiations possess both wave like and particle like properties, and
(ii) Experimental results regarding atomic spectra.
First, we will discuss about the duel nature of electromagnetic radiations. Experimental results regarding atomic spectra will be discussed in Section 2.4.
2.3.1 Wave Nature of Electromagnetic Radiation
In the mid-nineteenth century, physicists actively studied absorption and emission of radiation by heated objects. These are called thermal radiations. They tried to find out of what the thermal radiation is made. It is now a well-known fact that thermal radiations consist of electromagnetic waves of various frequencies or wavelengths. It is based on a number of modern concepts, which were unknown in the mid-nineteenth century. First active study of thermal radiation laws occured in the 1850’s and the theory of electromagnetic waves and the emission of such waves by accelerating charged particles was developed in the early 1870’s by James Clerk Maxwell, which was experimentally confirmed later by Heinrich Hertz. Here, we will learn some facts about electromagnetic radiations.
James Maxwell (1870) was the first to give a comprehensive explanation about the interaction between the charged bodies and the behaviour of electrical and magnetic fields on macroscopic level. He suggested that when electrically charged particle moves under accelaration, alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the forms of waves called electromagnetic waves or electromagnetic radiation.
Light is the form of radiation known from early days and speculation about its nature dates back to remote ancient times. In earlier days (Newton) light was supposed to be made of particles (corpuscules). It was only in the 19th century when wave nature of light was established.
* Classical mechanics is a theoretical science based on Newton’s laws of motion. It specifies the laws of motion of macroscopic objects.
Maxwell was again the first to reveal that light waves are associated with oscillating electric and magnetic character (Fig. 2.6). Although electromagnetic wave motion is complex in nature, we will consider here only a few simple properties.
(i) The oscillating electric and magnetic fields produced by oscillating charged particles are perpendicular to each other and both are perpendicular to the direction of propagation of the wave. Simplified picture of electromagnetic wave is shown in Fig. 2.6.
(ii) Unlike sound waves or waves produced in water, electromagnetic waves do not require medium and can move in vacuum.
(iii) It is now well established that there are many types of electromagnetic radiations, which differ from one another in wavelength (or frequency). These constitute what is called electromagnetic spectrum (Fig. 2.7). Different regions of the spectrum are identified by different names. Some examples are: radio frequency region around 106 Hz, used for broadcasting; microwave region around 1010 Hz used for radar; infrared region around 1013 Hz used for heating; ultraviolet region around 1016Hz a component of sun’s radiation. The small portion around 1015 Hz, is what is ordinarily called visible light. It is only this part which our eyes can see (or detect). Special instruments are required to detect non-visible radiation.
(iv) Different kinds of units are used to represent electromagnetic radiation.
These radiations are characterised by the properties, namely, frequency (ν) and wavelength (λ).
The SI unit for frequency (ν) is hertz
(Hz, s–1), after Heinrich Hertz. It is defined as the number of waves that pass a given point in one second.
Wavelength should have the units of length and as you know that the SI units of length is meter (m). Since electromagnetic radiation consists of different kinds of waves of much smaller wavelengths, smaller units are used. Fig.2.7 shows various types of electro-magnetic radiations which differ from one another in wavelengths and frequencies.
In vaccum all types of electromagnetic radiations, regardless of wavelength, travel at the same speed, i.e., 3.0 × 108 m s–1 (2.997925 × 108 m s–1, to be precise). This is called speed of light and is given the symbol ‘c‘. The frequency (ν ), wavelength (λ) and velocity of light (c) are related by the equation (2.5).
c = ν λ (2.5)
The other commonly used quantity specially in spectroscopy, is the wavenumber ( ). It is defined as the number of wavelengths per unit length. Its units are reciprocal of wavelength unit, i.e., m–1. However commonly used unit is cm–1 (not SI unit).
). It is defined as the number of wavelengths per unit length. Its units are reciprocal of wavelength unit, i.e., m–1. However commonly used unit is cm–1 (not SI unit).
Problem 2.3
The Vividh Bharati station of All India Radio, Delhi, broadcasts on a frequency of 1,368 kHz (kilo hertz). Calculate the wavelength of the electromagnetic radiation emitted by transmitter. Which part of the electromagnetic spectrum does it belong to?
Solution
The wavelength, λ, is equal to c/ν, where c is the speed of electromagnetic radiation in vacuum and ν is the frequency. Substituting the given values, we have
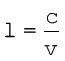

This is a characteristic radiowave wavelength.
Problem 2.4
The wavelength range of the visible spectrum extends from violet (400 nm) to red (750 nm). Express these wavelengths in frequencies (Hz). (1nm = 10–9 m)
Solution
Using equation 2.5, frequency of violet light
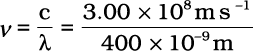
= 7.50 × 1014 Hz
Frequency of red light
ν = 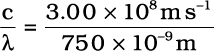 = 4.00 × 1014 Hz
= 4.00 × 1014 Hz
The range of visible spectrum is from
4.0 × 1014 to 7.5 × 1014 Hz in terms of frequency units.
Problem 2.5
Calculate (a) wavenumber and (b) frequency of yellow radiation having wavelength 5800 Å.
Solution
(a) Calculation of wavenumber (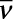 )
)
λ=5800Å = 5800 × 10–8 cm
= 5800 × 10–10 m
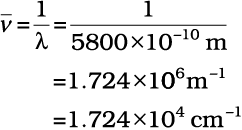
(b) Calculation of the frequency (ν )
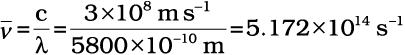
2.3.2 Particle Nature of Electromagnetic Radiation: Planck’s Quantum Theory
Some of the experimental phenomenon such as diffraction* and interference** can be explained by the wave nature of the electromagnetic radiation. However, following are some of the observations which could not be explained with the help of even the electromagentic theory of 19th century physics (known as classical physics):
(i) the nature of emission of radiation from hot bodies (black -body radiation)
(ii) ejection of electrons from metal surface when radiation strikes it (photoelectric effect)
(iii) variation of heat capacity of solids as a function of temperature
(iv) Line spectra of atoms with special reference to hydrogen.
These phenomena indicate that the system can take energy only in discrete amounts. All possible energies cannot be taken up or radiated.
It is noteworthy that the first concrete explanation for the phenomenon of the black body radiation mentioned above was given by Max Planck in 1900. Let us first try to understand this phenomenon, which is given below:
Hot objects emit electromagnetic radiations over a wide range of wavelengths. At high temperatures, an appreciable proportion of radiation is in the visible region of the spectrum. As the temperature is raised, a higher proportion of short wavelength (blue light) is generated. For example, when an iron rod is heated in a furnace, it first turns to dull red and then progressively becomes more and more red as the temperature increases. As this is heated further, the radiation emitted becomes
white and then becomes blue as the temperature becomes very high. This means that red radiation is most intense at a particular temperature and the blue radiation is more intense at another temperature. This means intensities of radiations of different wavelengths emitted by hot body depend upon its temperature. By late 1850’s it was known that objects made of different material and kept at different temperatures emit different amount of radiation. Also,






