Table of Contents

Unit 3
Classification of Elements and Periodicity in Properties

After studying this Unit, you will be able to
• appreciate how the concept of grouping elements in accordance to their properties led to the development of Periodic Table.
• understand the Periodic Law;
• understand the significance of atomic number and electronic configuration as the basis for periodic classification;
• name the elements with Z >100 according to IUPAC nomenclature;
• classify elements into s, p, d, f blocks and learn their main characteristics;
• recognise the periodic trends in physical and chemical properties of elements;
• compare the reactivity of elements and correlate it with their occurrence in nature;
• explain the relationship between ionization enthalpy and metallic character;
• use scientific vocabulary appropriately to communicate ideas related to certain important properties of atoms e.g., atomic/ ionic radii, ionization enthalpy, electron gain enthalpy, electronegativity, valence of elements.
The Periodic Table is arguably the most important concept in chemistry, both in principle and in practice. It is the everyday support for students, it suggests new avenues of research to professionals, and it provides a succinct organization of the whole of chemistry. It is a remarkable demonstration of the fact that the chemical elements are not a random cluster of entities but instead display trends and lie together in families. An awareness of the Periodic Table is essential to anyone who wishes to disentangle the world and see how it is built up from the fundamental building blocks of the chemistry, the chemical elements.
Glenn T. Seaborg
In this Unit, we will study the historical development of the Periodic Table as it stands today and the Modern Periodic Law. We will also learn how the periodic classification follows as a logical consequence of the electronic configuration of atoms. Finally, we shall examine some of the periodic trends in the physical and chemical properties of the elements.
3.1 WHY DO WE NEED TO CLASSIFY ELEMENTS ?
We know by now that the elements are the basic units of all types of matter. In 1800, only 31 elements were known. By 1865, the number of identified elements had more than doubled to 63. At present 114 elements are known. Of them, the recently discovered elements are man-made. Efforts to synthesise new elements are continuing. With such a large number of elements it is very difficult to study individually the chemistry of all these elements and their innumerable compounds individually. To ease out this problem, scientists searched for a systematic way to organise their knowledge by classifying the elements. Not only that it would rationalize known chemical facts about elements, but even predict new ones for undertaking further study.
3.2 GENESIS OF PERIODIC CLASSIFICATION
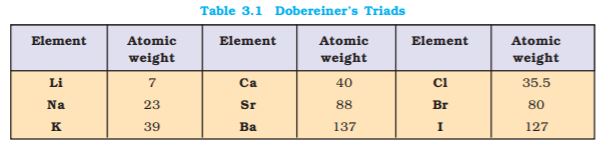
Classification of elements into groups and development of Periodic Law and Periodic Table are the consequences of systematising the knowledge gained by a number of scientists through their observations and experiments. The German chemist, Johann Dobereiner in early 1800’s was the first to consider the idea of trends among properties of elements. By 1829 he noted a similarity among the physical and chemical properties of several groups of three elements (Triads). In each case, he noticed that the middle element of each of the Triads had an atomic weight about half way between the atomic weights of the other two (Table 3.1). Also the properties of the middle element were in between those of the other
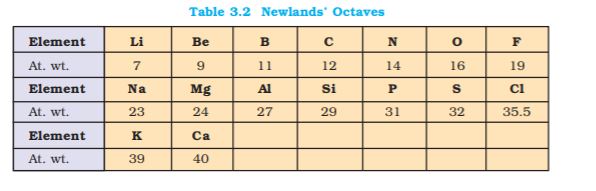
two members. Since Dobereiner’s relationship, referred to as the Law of Triads, seemed to work only for a few elements, it was dismissed as coincidence. The next reported attempt to classify elements was made by a French geologist, A.E.B. de Chancourtois in 1862. He arranged the then known elements in order of increasing atomic weights and made a cylindrical table of elements to display the periodic recurrence of properties. This also did not attract much attention. The English chemist, John Alexander Newlands in 1865 profounded the Law of Octaves. He arranged the elements in increasing order of their atomic weights and noted that every eighth element had properties similar to the first element (Table 3.2). The relationship was just like every eighth note that resembles the first in octaves of music. Newlands’s Law of Octaves seemed to be true only for elements up to calcium. Although his idea was not widely accepted at that time, he, for his work, was later awarded Davy Medal in 1887 by the Royal Society, London.
The Periodic Law, as we know it today owes its development to the Russian chemist, Dmitri Mendeleev (1834-1907) and the German chemist, Lothar Meyer (1830-1895). Working independently, both the chemists in 1869 proposed that on arranging elements in the increasing order of their atomic weights, similarities appear in physical and chemical properties at regular intervals. Lothar Meyer plotted the physical properties such as atomic volume, melting point and boiling point against atomic weight and obtained a periodically repeated pattern. Unlike Newlands, Lothar Meyer observed a change in length of that repeating pattern. By 1868, Lothar Meyer had developed a table of the elements that closely resembles the Modern Periodic Table. However, his work was not published until after the work of Dmitri Mendeleev, the scientist who is generally credited with the development of the Modern Periodic Table.
While Dobereiner initiated the study of periodic relationship, it was Mendeleev who was responsible for publishing the Periodic Law for the first time. It states as follows :
The properties of the elements are a periodic function of their atomic weights.
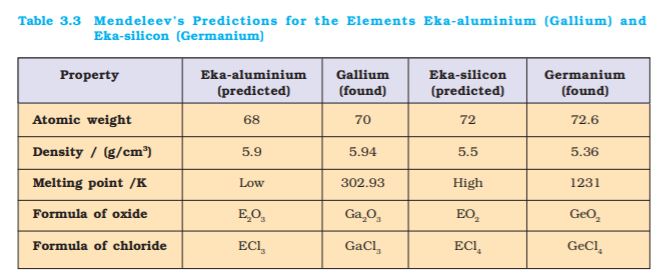
Mendeleev arranged elements in horizontal rows and vertical columns of a table in order of their increasing atomic weights in such a way that the elements with similar properties occupied the same vertical column or group. Mendeleev’s system of classifying elements was more elaborate than that of Lothar Meyer’s. He fully recognized the significance of periodicity and used broader range of physical and chemical properties to classify the elements. In particular, Mendeleev relied on the similarities in the empirical formulas and properties of the compounds formed by the elements. He realized that some of the elements did not fit in with his scheme of classification if the order of atomic weight was strictly followed. He ignored the order of atomic weights, thinking that the atomic measurements might be incorrect, and placed the elements with similar properties together. For example, iodine with lower atomic weight than that of tellurium (Group VI) was placed in Group VII along with fluorine, chlorine, bromine because of similarities in properties (Fig. 3.1). At the same time, keeping his primary aim of arranging the elements of similar properties in the same group, he proposed that some of the elements were still undiscovered and, therefore, left several gaps in the table. For example, both gallium and germanium were unknown at the time Mendeleev published his Periodic Table. He left the gap under aluminium and a gap under silicon, and called these elements Eka-Aluminium and Eka-Silicon. Mendeleev predicted not only the existence of gallium and germanium, but also described some of their general physical properties. These elements were discovered later. Some of the properties predicted by Mendeleev for these elements and those found experimentally are listed in Table 3.3.
The boldness of Mendeleev’s quantitative predictions and their eventual success made him and his Periodic Table famous. Mendeleev’s Periodic Table published in 1905 is shown in Fig. 3.1.
3.3 MODERN PERIODIC LAW AND THE PRESENT FORM OF THE PERIODIC TABLE
We must bear in mind that when Mendeleev developed his Periodic Table, chemists knew nothing about the internal structure of atom. However, the beginning of the 20th century witnessed profound developments in theories about sub-atomic particles. In 1913, the English physicist, Henry Moseley observed regularities in the characteristic X-ray spectra of the elements. A plot of 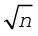 (where
(where is frequency of X-rays emitted) against atomic number (Z ) gave a straight line and not the plot of
is frequency of X-rays emitted) against atomic number (Z ) gave a straight line and not the plot of 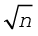 vs atomic mass. He thereby showed that the atomic number is a more fundamental property of an element than its atomic mass. Mendeleev’s Periodic Law was, therefore, accordingly modified. This is known as the Modern Periodic Law and can be stated as :
vs atomic mass. He thereby showed that the atomic number is a more fundamental property of an element than its atomic mass. Mendeleev’s Periodic Law was, therefore, accordingly modified. This is known as the Modern Periodic Law and can be stated as :
The physical and chemical properties of the elements are periodic functions of their atomic numbers.
The Periodic Law revealed important analogies among the 94 naturally occurring elements (neptunium and plutonium like actinium and protoactinium are also found in pitch blende – an ore of uranium). It stimulated renewed interest in Inorganic Chemistry and has carried into the present with the creation of artificially produced short-lived elements.
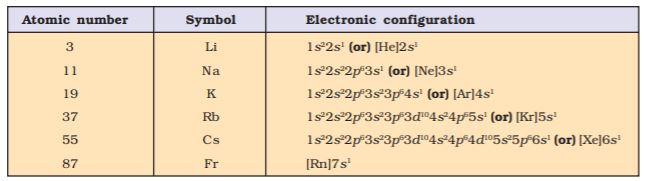
You may recall that the atomic number is equal to the nuclear charge (i.e., number of protons) or the number of electrons in a neutral atom. It is then easy to visualize the significance of quantum numbers and electronic configurations in periodicity of elements. In fact, it is now recognized that the Periodic Law is essentially the consequence of the periodic variation in electronic configurations, which indeed determine the physical and chemical properties of elements and their compounds.
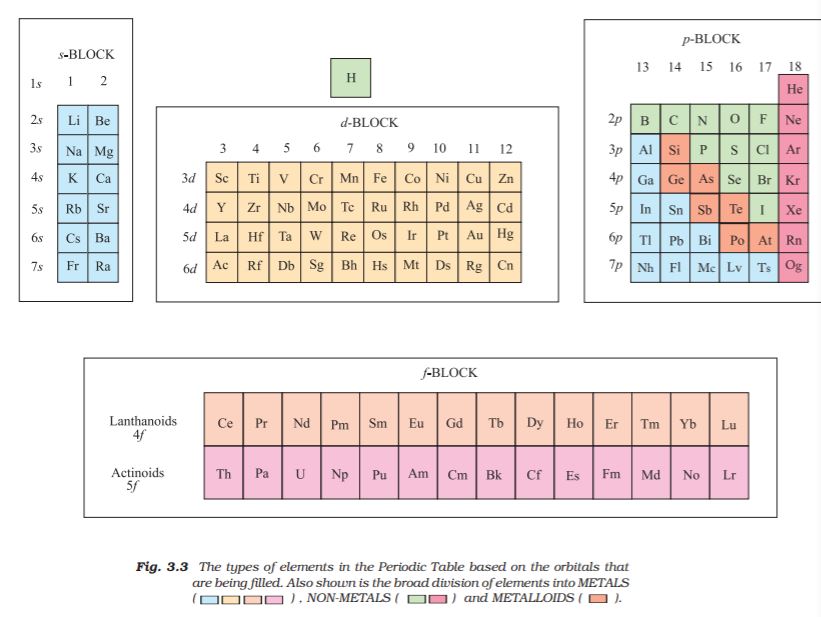
Numerous forms of Periodic Table have been devised from time to time. Some forms emphasise chemical reactions and valence, whereas others stress the electronic configuration of elements. A modern version, the so-called “long form” of the Periodic Table of the elements (Fig. 3.2), is the most convenient and widely used. The horizontal rows (which Mendeleev called series) are called periods and the vertical columns, groups. Elements having similar outer electronic configurations in their atoms are arranged in vertical columns, referred to as groups or families. According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 replacing the older notation of groups IA … VIIA, VIII, IB … VIIB and 0.
There are altogether seven periods. The period number corresponds to the highest principal quantum number (n) of the elements in the period. The first period contains 2 elements. The subsequent periods consists of 8, 8, 18, 18 and 32 elements, respectively. The seventh period is incomplete and like the sixth period would have a theoretical maximum (on the basis of quantum numbers) of 32 elements. In this form of the Periodic Table, 14 elements of both sixth and seventh periods (lanthanoids and actinoids, respectively) are placed in separate panels at the bottom*.
3.4 NOMENCLATURE OF ELEMENTS WITH ATOMIC NUMBERS > 100
The naming of the new elements had been traditionally the privilege of the discoverer (or discoverers) and the suggested name was ratified by the IUPAC. In recent years this has led to some controversy. The new elements with very high atomic numbers are so unstable that only minute quantities, sometimes only a few atoms of them are obtained. Their synthesis and characterisation, therefore, require highly sophisticated costly equipment and laboratory. Such work is carried out with competitive spirit only in some laboratories in the world. Scientists, before collecting the reliable data on the new element, at times get tempted to claim for its discovery. For example, both American and Soviet scientists claimed credit for discovering element 104. The Americans named it Rutherfordium whereas Soviets named it Kurchatovium. To avoid such problems, the IUPAC has made recommendation that until a new element’s discovery is proved, and its name is officially recognised, a systematic nomenclature be derived directly from the atomic number of the element using the numerical roots for 0 and numbers 1-9. These are shown in Table 3.4. The roots are put together in order of digits which make up the atomic number and “ium” is added at the end. The IUPAC names for elements with Z above 100 are shown in
Table 3.5.
Thus, the new element first gets a temporary name, with symbol consisting of three letters. Later permanent name and symbol are given by a vote of IUPAC representatives from each country. The permanent name might reflect the country (or state of the country) in which the element was discovered, or pay tribute to a notable scientist. As of now, elements with atomic numbers up to 118 have been discovered. Official names of all elements have been announced by IUPAC.
* Glenn T. Seaborg’s work in the middle of the 20th century starting with the discovery of plutonium in 1940, followed by those of all the transuranium elements from 94 to 102 led to reconfiguration of the periodic table placing the actinoids below the lanthanoids. In 1951, Seaborg was awarded the Nobel Prize in chemistry for his work. Element 106 has been named Seaborgium (Sg) in his honour.


Fig. 3.2 Long form of the Periodic Table of the Elements with their atomic numbers and ground state outer electronic configurations. The groups are numbered 1-18 in accordance with the 1984 IUPAC recommendations. This notation replaces the old numbering scheme of IA–VIIA, VIII, IB–VIIB and 0 for the elements.
Problem 3.1
What would be the IUPAC name and symbol for the element with atomic number 120?
Solution
From Table 3.4, the roots for 1, 2 and 0 are un, bi and nil, respectively.
Hence, the symbol and the namerespectively are Ubn and unbinilium.
3.5 ELECTRONIC CONFIGURATIONS OF ELEMENTS AND THE PERIODIC TABLE
In the preceding unit we have learnt that an electron in an atom is characterised by a set of four quantum numbers, and the principal quantum number (n ) defines the main energy level known as shell. We have also studied about the filling of electrons into different subshells, also referred to as orbitals (s, p, d, f) in an atom. The distribution of electrons into orbitals of an atom is called its electronic configuration. An element’s location in the Periodic Table reflects the quantum numbers of the last orbital filled. In this section we will observe a direct connection between the electronic configurations of the elements and the long form of the Periodic Table.
(a) Electronic Configurations in Periods
The period indicates the value of n for the outermost or valence shell. In other words, successive period in the Periodic Table is associated with the filling of the next higher principal energy level (n = 1, n = 2, etc.). It can be readily seen that the number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled. The first period (n = 1) starts with the filling of the lowest level (1s) and therefore has two elements — hydrogen (ls1) and helium (ls2) when the first shell (K) is completed. The second period (n = 2) starts with lithium and the third electron enters the 2s orbital. The next element, beryllium has four electrons and has the electronic configuration 1s22s2. Starting from the next element boron, the 2p orbitals are filled with electrons when the L shell is completed at neon (2s22p6). Thus there are
8 elements in the second period. The third period (n = 3) begins at sodium, and the added electron enters a 3s orbital. Successive filling of 3s and 3p orbitals gives rise to the third period of 8 elements from sodium to argon. The fourth period (n = 4) starts at potassium, and the added electrons fill up the 4s orbital. Now you may note that before the 4p orbital is filled, filling up of 3d orbitals becomes energetically favourable and we come across the so called 3d transition series of elements. This starts from scandium (Z = 21) which has the electronic configuration 3d14s2. The 3d orbitals are filled at zinc (Z=30) with electronic configuration 3d104s2 . The fourth period ends at krypton with the filling up of the 4p orbitals. Altogether we have 18 elements in this fourth period. The fifth period (n = 5) beginning with rubidium is similar to the fourth period and contains the 4d transition series starting at yttrium
(Z = 39). This period ends at xenon with the filling up of the 5p orbitals. The sixth period (n = 6) contains 32 elements and successive electrons enter 6s, 4f, 5d and 6p orbitals, in the order — filling up of the 4f orbitals begins with cerium (Z = 58) and ends at lutetium (Z = 71) to give the 4f-inner transition series which is called the lanthanoid series. The seventh period (n = 7) is similar to the sixth period with the successive filling up of the 7s, 5f, 6d and 7p orbitals and includes most of the man-made radioactive elements. This period will end at the element with atomic number 118 which would belong to the noble gas family. Filling up of the 5f orbitals after actinium (Z = 89) gives the 5f-inner transition series known as the actinoid series. The 4f- and 5f-inner transition series of elements are placed separately in the Periodic Table to maintain its structure and to preserve the principle of classification by keeping elements with similar properties in a single column.
Problem 3.2
How would you justify the presence of 18 elements in the 5th period of the Periodic Table?
Solution
When n = 5, l = 0, 1, 2, 3. The order in which the energy of the available orbitals 4d, 5s and 5p increases is 5s < 4d < 5p. The total number of orbitals available are 9. The maximum number of electrons that can be accommodated is 18; and therefore 18 elements are there in the 5th period.
(b) Groupwise Electronic Configurations
Elements in the same vertical column or group have similar valence shell electronic configurations, the same number of electrons in the outer orbitals, and similar properties. For example, the Group 1 elements (alkali metals) all have ns1 valence shell electronic configuration as shown below.
Thus it can be seen that the properties of an element have periodic dependence upon its atomic number and not on relative atomic mass.
3.6 ELECTRONIC CONFIGURATIONS AND TYPES OF ELEMENTS:
s-, p-, d-, f- BLOCKS
The aufbau (build up) principle and the electronic configuration of atoms provide a theoretical foundation for the periodic classification. The elements in a vertical column of the Periodic Table constitute a group or family and exhibit similar chemical behaviour. This similarity arises because these elements have the same number and same distribution of electrons in their outermost orbitals. We can classify the elements into four blocks viz.,
s-block, p-block, d-block and f-block depending on the type of atomic orbitals that are being filled with electrons. This is illustrated in Fig. 3.3. We notice two exceptions to this categorisation. Strictly, helium belongs to the
s-block but its positioning in the p-block along with other group 18 elements is justified because it has a completely filled valence shell (1s2) and as a result, exhibits properties characteristic of other noble gases. The other exception is hydrogen. It has only one s-electron and hence can be placed in group 1 (alkali metals). It can also gain an electron to achieve a noble gas arrangement and hence it can behave similar to a group 17 (halogen family) elements. Because it is a special case, we shall place hydrogen separately at the top of the Periodic Table as shown in Fig. 3.2 and Fig. 3.3. We will briefly discuss the salient features of the four types of elements marked in the Periodic Table. More about these elements will be discussed later. During the description of their features certain terminology has been used which has been classified in section 3.7.
3.6.1 The s-Block Elements
The elements of Group 1 (alkali metals) and Group 2 (alkaline earth metals) which have ns1 and ns2 outermost electronic configuration belong to the s-Block Elements. They are all reactive metals with low ionization enthalpies. They lose the outermost electron(s) readily to form 1+ ion (in the case of alkali metals) or 2+ ion (in the case of alkaline earth metals). The metallic character and the reactivity increase as we go down the group. Because of high reactivity they are never found pure in nature. The compounds of the s-block elements, with the exception of those of lithium and beryllium are predominantly ionic.
3.6.2 The p-Block Elements
The p-Block Elements comprise those belonging to Group 13 to 18 and these together with the s-Block Elements are called the Representative Elements or Main Group Elements. The outermost electronic configuration varies from ns2np1 to ns2np6 in each period. At the end of each period is a noble gas element with a closed valence shell ns2np6 configuration. All the orbitals in the valence shell of the noble gases are completely filled by electrons and it is very difficult to alter this stable arrangement by the addition or removal of electrons. The noble gases thus exhibit very low chemical reactivity. Preceding the noble gas family are two chemically important groups of non-metals. They are the halogens (Group 17) and the chalcogens (Group 16). These two groups of elements have highly negative electron gain enthalpies and readily add one or two electrons respectively to attain the stable noble gas configuration. The non-metallic character increases as we move from left to right across a period and metallic character increases as we go down the group.
3.6.3 The d-Block Elements (Transition Elements)
These are the elements of Group 3 to 12 in the centre of the Periodic Table. These are characterised by the filling of inner d orbitals by electrons and are therefore referred to as
d-Block Elements. These elements have the general outer electronic configuration
(n-1)d1-10ns0-2 . They are all metals. They mostly form coloured ions, exhibit variable valence (oxidation states), paramagnetism and oftenly used as catalysts. However, Zn, Cd and Hg which have the electronic configuration,
(n-1) d10ns2 do not show most of the properties of transition elements. In a way, transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14 and thus take their familiar name “Transition Elements”.
3.6.4 The f-Block Elements
(Inner-Transition Elements)
The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) – Lu(Z = 71) and Actinoids, Th(Z = 90) – Lr (Z = 103) are characterised by the outer electronic configuration (n-2)f1-14
(n-1)d0–1ns2. The last electron added to each element is filled in f- orbital. These two series of elements are hence called the Inner-Transition Elements (f-Block Elements). They are all metals. Within each series, the properties of the elements are quite similar. The chemistry of the early actinoids is more complicated than the corresponding lanthanoids, due to the large number of oxidation states possible for these actinoid elements. Actinoid elements are radioactive. Many of the actinoid elements have been made only in nanogram quantities or even less by nuclear reactions and their chemistry is not fully studied. The elements after uranium are called Transuranium Elements.
Problem 3.3
The elements Z = 117 and 120 have not yet been discovered. In which family / group would you place these elements and also give the electronic configuration in each case.
Solution
We see from Fig. 3.2, that element with Z = 117, would belong to the halogen family (Group 17) and the electronic configuration would be [Rn] 5f146d107s27p5. The element with Z = 120, will be placed in Group 2 (alkaline earth metals), and will have the electronic configuration [Uuo]8s2.
3.6.5 Metals, Non-metals and Metalloids
In addition to displaying the classification of elements into s-, p-, d-, and f-blocks, Fig. 3.3 shows another broad classification of elements based on their properties. The elements can be divided into Metals and Non-Metals. Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table. Metals are usually solids at room temperature [mercury is an exception; gallium and caesium also have very low melting points (303K and 302K, respectively)]. Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires). In contrast, non-metals are located at the top right hand side of the Periodic Table. In fact, in a horizontal row, the property of elements change from metallic on the left to non-metallic on the right. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). They are poor conductors of heat and electricity. Most non-metallic solids are brittle and are neither malleable nor ductile. The elements become more metallic as we go down a group; the non-metallic character increases as one goes from left to right across the Periodic Table. The change from metallic to non-metallic character is not abrupt as shown by the thick zig-zag line in Fig. 3.3. The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) bordering this line and running diagonally across the Periodic Table show properties that are characteristic of both metals and non-metals. These elements are called Semi-metals or Metalloids.
Problem 3.4
Considering the atomic number and position in the periodic table, arrange the following elements in the increasing order of metallic character : Si, Be, Mg, Na, P.
Solution
Metallic character increases down a group and decreases along a period as we move from left to right. Hence the order of increasing metallic character is: P < Si < Be < Mg < Na.
3.7 PERIODIC TRENDS IN PROPERTIES OF ELEMENTS
There are many observable patterns in the physical and chemical properties of elements as we descend in a group or move across a period in the Periodic Table. For example, within a period, chemical reactivity tends to be high in Group 1 metals, lower in elements towards the middle of the table, and increases to a maximum in the Group 17 non-metals. Likewise within a group of representative metals (say alkali metals) reactivity increases on moving down the group, whereas within a group of non-metals (say halogens), reactivity decreases down the group. But why do the properties of elements follow these trends? And how can we explain periodicity? To answer these questions, we must look into the theories of atomic structure and properties of the atom. In this section we shall discuss the periodic trends in certain physical and chemical properties and try to explain them in terms of number of electrons and energy levels.
3.7.1 Trends in Physical Properties
There are numerous physical properties of elements such as melting and boiling points, heats of fusion and vaporization, energy of atomization, etc. which show periodic variations. However, we shall discuss the periodic trends with respect to atomic and ionic radii, ionization enthalpy, electron gain enthalpy and electronegativity.
(a) Atomic Radius
You can very well imagine that finding the size