Table of Contents
Chapter Two
Units and Measurement
2.1 Introduction
2.2 The international system of units
2.3 Measurement of length
2.4 Measurement of mass
2.5 Measurement of time
2.6 Accuracy, precision of instruments and errors in measurement
2.7 Significant figures
2.8 Dimensions of physical quantities
2.9 Dimensional formulae and dimensional equations
2.10 Dimensional analysis and its applications
Summary
Exercises
Additional exercises
2.1 Introduction
Measurement of any physical quantity involves comparison with a certain basic, arbitrarily chosen, internationally accepted reference standard called unit. The result of a measurement of a physical quantity is expressed by a number (or numerical measure) accompanied by a unit. Although the number of physical quantities appears to be very large, we need only a limited number of units for expressing all the physical quantities, since they are inter-related with one another. The units for the fundamental or base quantities are called fundamental or base units. The units of all other physical quantities can be expressed as combinations of the base units. Such units obtained for the derived quantities are called derived units. A complete set of these units, both the base units and derived units, is known as the system of units.
2.2 The International System of Units
In earlier time scientists of different countries were using different systems of units for measurement. Three such systems, the CGS, the FPS (or British) system and the MKS system were in use extensively till recently.
The base units for length, mass and time in these systems were as follows :
• In CGS system they were centimetre, gram and second respectively.
• In FPS system they were foot, pound and second respectively.
• In MKS system they were metre, kilogram and second respectively.
The system of units which is at present internationally accepted for measurement is the Système Internationale
d’ Unites (French for International System of Units), abbreviated as SI. The SI, with standard scheme of symbols, units and abbreviations, developed by the Bureau International des Poids et measures (The International Bureau of weights and measures, BIPM) in 1971 were recently revised by the General Conference on weights and measures in November 2018. The scheme is now for international usage in scientific, technical, industrial and commercial work. Because SI units used decimal system, conversions within the system are quite simple and convenient. We shall follow the SI units in this book.
In SI, there are seven base units as given in Table 2.1. Besides the seven base units, there are two more units that are defined for (a) plane angle dθ as the ratio of length of arc ds to the radius r and (b) solid angle dΩ as the ratio of the intercepted area dA of the spherical surface, described about the apex O as the centre, to the square of its radius r, as shown in Fig. 2.1(a) and (b) respectively. The unit for plane angle is radian with the symbol rad and the unit for the solid angle is steradian with the symbol sr. Both these are dimensionless quantities.
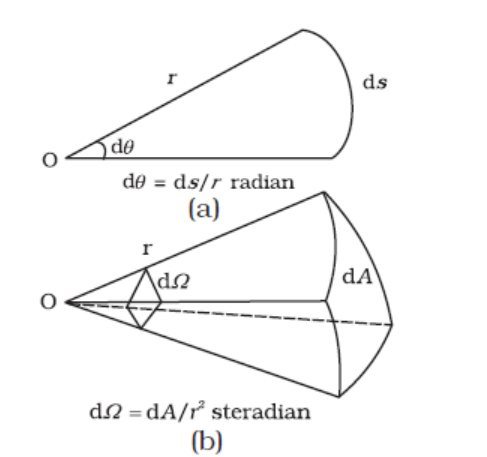
Fig. 2.1 Description of (a) plane angle dθ and (b) solid angle
Table 2.1 SI Base Quantities and Units*
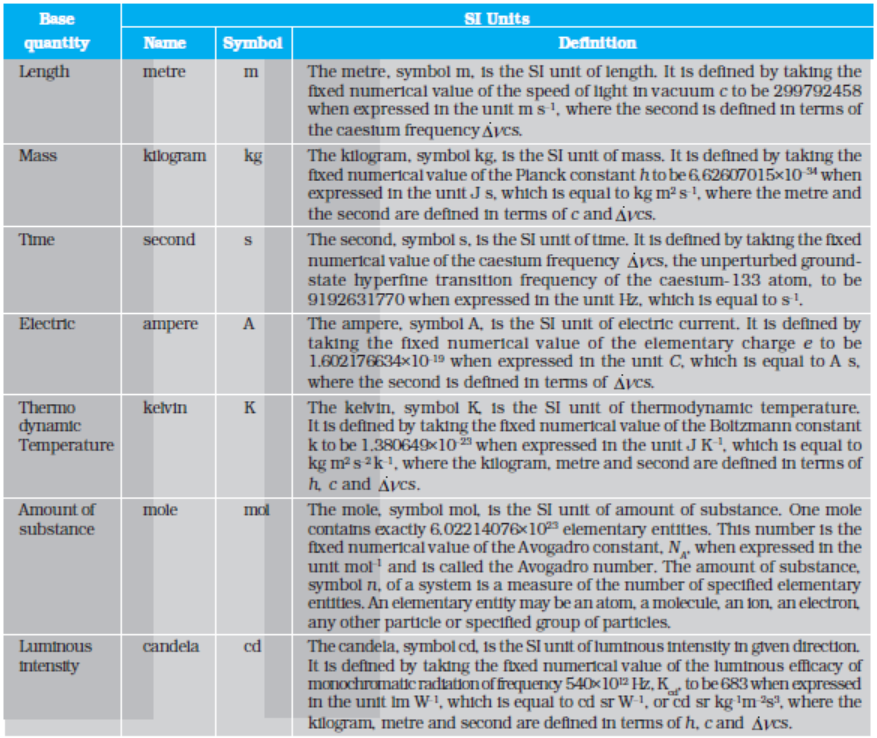
* The values mentioned here need not be remembered or asked in a test. They are given here only to indicate the extent of accuracy to which they are measured. With progress in technology, the measuring techniques get improved leading to measurements with greater precision. The definitions of base units are revised to keep up with this progress.
Table 2.2 Some units retained for general use (Though outside SI)
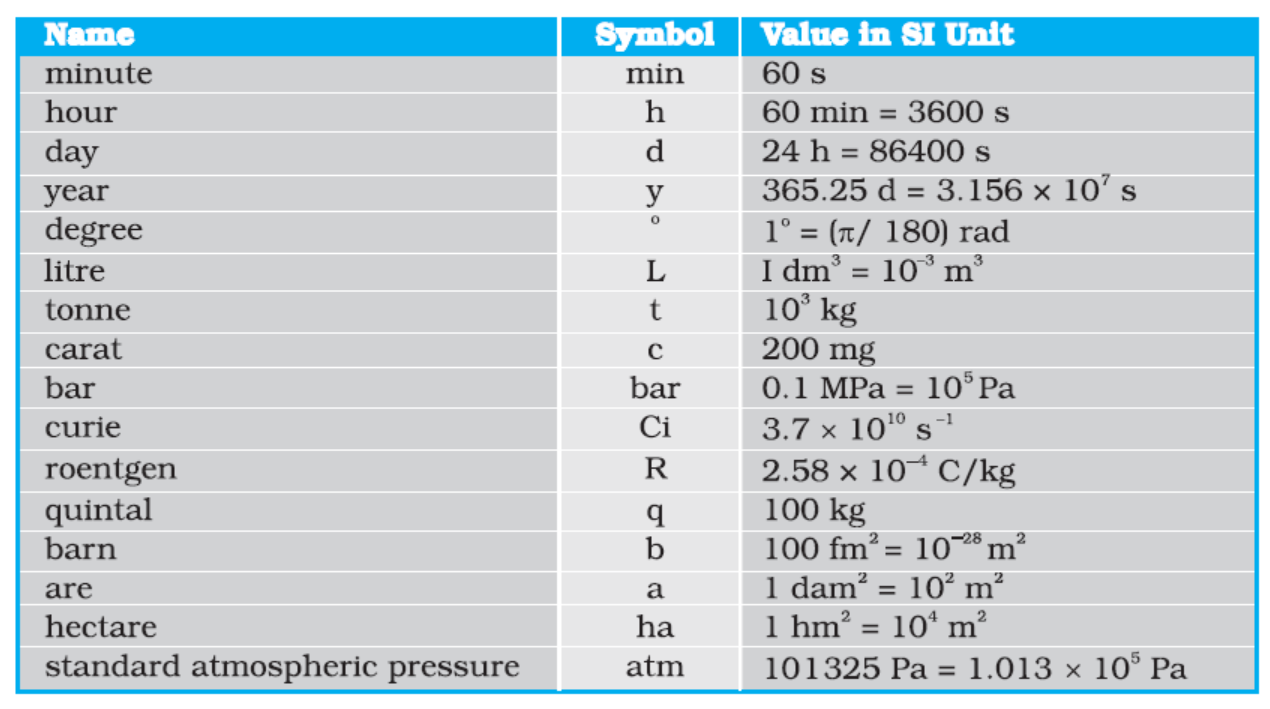
Note that when mole is used, the elementary entities must be specified. These entities may be atoms, molecules, ions, electrons, other particles or specified groups of such particles.
We employ units for some physical quantities that can be derived from the seven base units (Appendix A 6). Some derived units in terms of the SI base units are given in (Appendix A 6.1). Some SI derived units are given special names (Appendix A 6.2 ) and some derived SI units make use of these units with special names and the seven base units (Appendix A 6.3). These are given in Appendix A 6.2 and A 6.3 for your ready reference. Other units retained for general use are given in Table 2.2.
Common SI prefixes and symbols for multiples and sub-multiples are given in Appendix A2. General guidelines for using symbols for physical quantities, chemical elements and nuclides are given in Appendix A7 and those for SI units and some other units are given in Appendix A8 for your guidance and ready reference.
2.3 Measurement of Length
You are already familiar with some direct methods for the measurement of length. For example, a metre scale is used for lengths from 10–3 m to 102 m. A vernier callipers is used for lengths to an accuracy of 10–4 m. A screw gauge and a spherometer can be used to measure lengths as less as to 10–5 m. To measure lengths beyond these ranges, we make use of some special indirect methods.
2.3.1 Measurement of Large Distances
Large distances such as the distance of a planet or a star from the earth cannot be measured directly with a metre scale. An important method in such cases is the parallax method.
When you hold a pencil in front of you against some specific point on the background (a wall) and look at the pencil first through your left eye A (closing the right eye) and then look at the pencil through your right eye B (closing the left eye), you would notice that the position of the pencil seems to change with respect to the point on the wall. This is called parallax. The distance between the two points of observation is called the basis. In this example, the basis is the distance between the eyes.
To measure the distance D of a far away planet S by the parallax method, we observe it from two different positions (observatories) A and B on the Earth, separated by distance AB = b at the same time as shown in Fig. 2.2. We measure the angle between the two directions along which the planet is viewed at these two points. The ∠ASB in Fig. 2.2 represented by symbol θ is called the parallax angle or parallactic angle.
As the planet is very far away,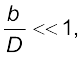 and therefore,
and therefore,  is very small. Then we approximately take AB as an arc of length b of a circle with centre at S and the distance D as the radius AS = BS so that AB = b = D θ where θ is in radians.
is very small. Then we approximately take AB as an arc of length b of a circle with centre at S and the distance D as the radius AS = BS so that AB = b = D θ where θ is in radians.
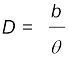 (2.1)
(2.1)
Having determined D, we can employ a similar method to determine the size or angular diameter of the planet. If d is the diameter of the planet and α the angular size of the planet (the angle subtended by d at the earth), we have
α = d/D (2.2)
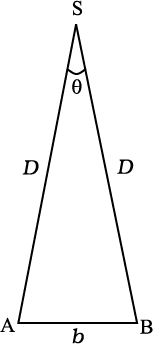
Fig. 2.2 Parallax method.
Example 2.1 Calculate the angle of (a) 10 (degree) (b) 1′ (minute of arc or arcmin) and (c) 1″(second of arc or arc second) in radians. Use 3600=2π rad, 10=60′ and 1′ = 60 ″
Answer (a) We have 3600 = 2π rad
10 = (π /180) rad = 1.745×10–2 rad
(b) 10 = 60′ = 1.745×10–2 rad
1′ = 2.908×10–4 rad  2.91×10–4 rad
2.91×10–4 rad
(c) 1′ = 60″ = 2.908×10–4 rad
1″ = 4.847×10–4 rad  4.85×10–6 rad
4.85×10–6 rad
Example 2.2 A man wishes to estimate the distance of a nearby tower from him. He stands at a point A in front of the tower C and spots a very distant object O in line with AC. He then walks perpendicular to AC up to B, a distance of 100 m, and looks at O and C again. Since O is very distant, the direction BO is practically the same as AO; but he finds the line of sight of C shifted from the original line of sight by an angle θ = 400 (θ is known as ‘parallax’) estimate the distance of the tower C from his original position A.
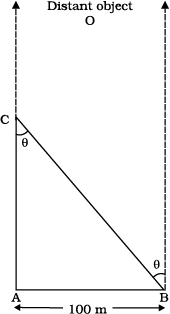
Fig. 2.3
Answer We have, parallax angle θ = 400
From Fig. 2.3, AB = AC tan θ
AC = AB/tanθ = 100 m/tan 400
= 100 m/0.8391 = 119 m
Example 2.3 The moon is observed from two diametrically opposite points A and B on Earth. The angle θ subtended at the moon by the two directions of observation is 1o 54′. Given the diameter of the Earth to be about 1.276 × 107 m, compute the distance of the moon from the Earth.
Answer We have θ = 1° 54′ = 114′
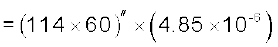 rad
rad 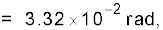
since 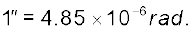
Also 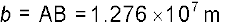
Hence from Eq. (2.1), we have the earth-moon distance, 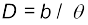

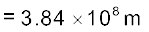
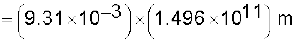
Example 2.4 The Sun’s angular diameter is measured to be 1920′′. The distance D of the Sun from the Earth is 1.496 × 1011 m. What is the diameter of the Sun?
Answer Sun’s angular diameter α
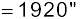
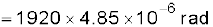
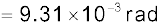
Sun’s diameter
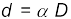
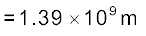
2.3.2 Estimation of Very Small Distances: Size of a Molecule
To measure a very small size, like that of a molecule (10–8 m to 10–10 m), we have to adopt special methods. We cannot use a screw gauge or similar instruments. Even a microscope has certain limitations. An optical microscope uses visible light to ‘look’ at the system under investigation. As light has wave like features, the resolution to which an optical microscope can be used is the wavelength of light (A detailed explanation can be found in the Class XII Physics textbook). For visible light the range of wavelengths is from about 4000 Å to 7000 Å
(1 angstrom = 1 Å = 10-10 m). Hence an optical microscope cannot resolve particles with sizes smaller than this. Instead of visible light, we can use an electron beam. Electron beams can be focussed by properly designed electric and magnetic fields. The resolution of such an electron microscope is limited finally by the fact that electrons can also behave as waves ! (You will learn more about this in class XII). The wavelength of an electron can be as small as a fraction of an angstrom. Such electron microscopes with a resolution of 0.6 Å have been built. They can almost resolve atoms and molecules in a material. In recent times, tunnelling microscopy has been developed in which again the limit of resolution is better than an angstrom. It is possible to estimate the sizes of molecules.
A simple method for estimating the molecular size of oleic acid is given below. Oleic acid is a soapy liquid with large molecular size of the order of 10–9 m.
The idea is to first form mono-molecular layer of oleic acid on water surface.
We dissolve 1 cm3 of oleic acid in alcohol to make a solution of 20 cm3. Then we take 1 cm3 of this solution and dilute it to 20 cm3, using alcohol. So, the concentration of the solution is equal to 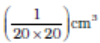 of oleic acid/cm3 of solution. Next we lightly sprinkle some lycopodium powder on the surface of water in a large trough and we put one drop of this solution in the water. The oleic acid drop spreads into a thin, large and roughly circular film of molecular thickness on water surface. Then, we quickly measure the diameter of the thin film to get its area A. Suppose we have dropped n drops in the water. Initially, we determine the approximate volume of each drop (V cm3).
of oleic acid/cm3 of solution. Next we lightly sprinkle some lycopodium powder on the surface of water in a large trough and we put one drop of this solution in the water. The oleic acid drop spreads into a thin, large and roughly circular film of molecular thickness on water surface. Then, we quickly measure the diameter of the thin film to get its area A. Suppose we have dropped n drops in the water. Initially, we determine the approximate volume of each drop (V cm3).
Volume of n drops of solution
= nV cm3
Amount of oleic acid in this solution
= 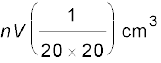
This solution of oleic acid spreads very fast on the surface of water and forms a very thin layer of thickness t. If this spreads to form a film of area A cm2, then the thickness of the film
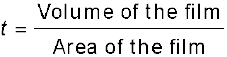
or, 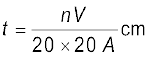 (2.3)
(2.3)
If we assume that the film has mono-molecular thickness, then this becomes the size or diameter of a molecule of oleic acid. The value of this thickness comes out to be of the order of 10–9 m.
Example 2.5 If the size of a nucleus (in the range of 10–15 to 10–14 m) is scaled up to the tip of a sharp pin, what roughly is the size of an atom ? Assume tip of the pin to be in the range 10–5m to 10–4m.
Answer The size of a nucleus is in the range of 10–15 m and 10–14 m. The tip of a sharp pin is taken to be in the range of 10–5 m and 10–4 m. Thus we are scaling up by a factor of 1010. An atom roughly of size 10–10 m will be scaled up to a size of 1 m. Thus a nucleus in an atom is as small in size as the tip of a sharp pin placed at the centre of a sphere of radius about a metre long.
2.3.3 Range of Lengths
The sizes of the objects we come across in the universe vary over a very wide range. These may vary from the size of the order of 10–14 m of the tiny nucleus of an atom to the size of the order of 1026 m of the extent of the observable universe. Table 2.3 gives the range and order of lengths and sizes of some of these objects.
We also use certain special length units for short and large lengths. These are
1 fermi = 1 f = 10–15 m
1 angstrom = 1 Å = 10–10 m
1 astronomical unit = 1 AU (average distance of the Sun from the Earth)
= 1.496 × 1011 m
1 light year = 1 ly = 9.46 × 1015 m (distance that light travels with velocity of
3 × 108 m s–1 in 1 year)
1 parsec = 3.08 × 1016 m (Parsec is the distance at which average radius of earth’s orbit subtends an angle of 1 arc second)
2.4 Measurement of mass
Mass is a basic property of matter. It does not depend on the temperature, pressure or location of the object in space. The SI unit of mass is kilogram (kg). It is defined by taking the fixed numerical value of the Plank Constant h to be 6.62607015×10–34 when expressed in the unit of Js which is equal to kg m2s–1, where the
metre and the second are defined is terms of C
and
 cs.
cs.
While dealing with atoms and molecules, the kilogram is an inconvenient unit. In this case, there is an important standard unit of mass, called the unified atomic mass unit (u), which has been established for expressing the mass of atoms as
1 unified atomic mass unit = 1u
= (1/12) of the mass of an atom of carbon-12 isotope  including the mass of electrons
including the mass of electrons
= 1.66 × 10–27 kg
Mass of commonly available objects can be determined by a common balance like the one used in a grocery shop. Large masses in the universe like planets, stars, etc., based on Newton’s law of gravitation can be measured by using gravitational method (See Chapter 8). For measurement of small masses of atomic/sub-atomic particles etc., we make use of mass spectrograph in which radius of the trajectory is proportional to the mass of a charged particle moving in uniform electric and magnetic field.
2.4.1 Range of Masses
The masses of the objects, we come across in the universe, vary over a very wide range. These may vary from tiny mass of the order of 10-30 kg of an electron to the huge mass of about 1055 kg of the known universe. Table 2.4 gives the range and order of the typical masses of various objects.
Table 2.3 Range and order of lengths
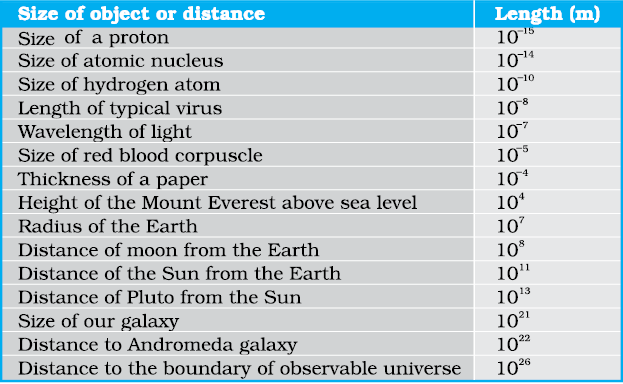
Table 2.4 Range and order of masses

2.5 Measurement of time
To measure any time interval we need a clock. We now use an atomic standard of time, which is based on the periodic vibrations produced in a cesium atom. This is the basis of the caesium clock, sometimes called atomic clock, used in the national standards. Such standards are available in many laboratories. In the caesium atomic clock, the second is taken as the time needed for 9,192,631,770 vibrations of the radiation corresponding to the transition between the two hyperfine levels of the ground state of caesium-133 atom. The vibrations of the caesium atom regulate the rate of this caesium atomic clock just as the vibrations of a balance wheel regulate an ordinary wristwatch or the vibrations of a small quartz crystal regulate a quartz wristwatch.
The caesium atomic clocks are very accurate. In principle they provide portable standard. The national standard of time interval ‘second’ as well as the frequency is maintained through four cesium atomic clocks. A caesium atomic clock is used at the National Physical Laboratory (NPL), New Delhi to maintain the Indian standard of time.
In our country, the NPL has the responsibility of maintenance and improvement of physical standards, including that of time, frequency, etc. Note that the Indian Standard Time (IST) is linked to this set of atomic clocks. The efficient caesium atomic clocks are so accurate that they impart the uncertainty in time realisation as
± 1 × 10–15, i.e. 1 part in 1015. This implies that the uncertainty gained over time by such a device is less than 1 part in 1015; they lose or gain no more than 32 µs in one year. In view of the tremendous accuracy in time measurement, the SI unit of length has been expressed in terms the path length light travels in certain interval of time (1/299, 792, 458 of a second) (Table 2.1).
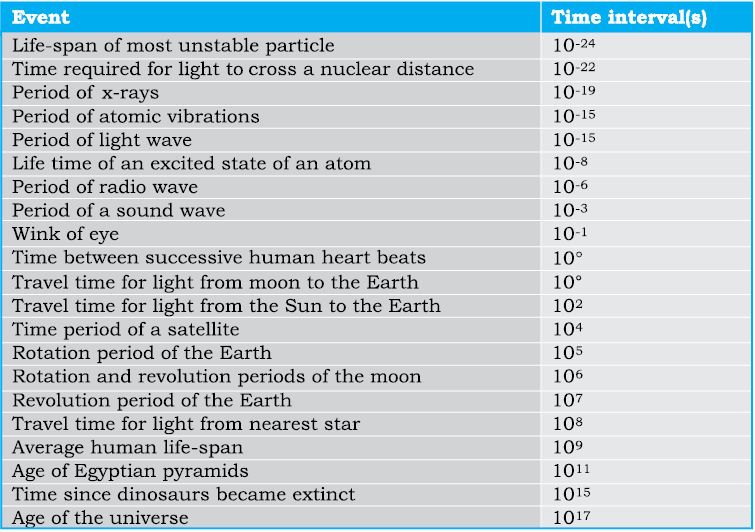
The time interval of events that we come across in the universe vary over a very wide range. Table 2.5 gives the range and order of some typical time intervals.
You may notice that there is an interesting coincidence between the numbers appearing in Tables 2.3 and 2.5. Note that the ratio of the longest and shortest lengths of objects in our universe is about 1041. Interestingly enough, the ratio of the longest and shortest time intervals associated with the events and objects in our universe is also about 1041. This number, 1041 comes up again in Table 2.4, which lists typical masses of objects. The ratio of the largest and smallest masses of the objects in our universe is about (1041)2. Is this a curious coincidence between these large numbers purely accidental ?
2.6 Accuracy, precision of instruments and errors in measurement
Measurement is the foundation of all experimental science and technology. The result of every measurement by any measuring instrument contains some uncertainty. This uncertainty is called error. Every calculated quantity which is based on measured values, also has an error. We shall distinguish between two terms: accuracy and precision. The accuracy of a measurement is a measure of how close the measured value is to the true value of the quantity. Precision tells us to what resolution or limit the quantity is measured.
The accuracy in measurement may depend on several factors, including the limit or the resolution of the measuring instrument. For example, suppose the true value of a certain length is near 3.678 cm. In one experiment, using a measuring instrument of resolution 0.1 cm, the measured value is found to be 3.5 cm, while in another experiment using a measuring device of greater resolution, say 0.01 cm, the length is determined to be 3.38 cm. The first measurement has more accuracy (because it is closer to the true value) but less precision (its resolution is only 0.1 cm), while the second measurement is less accurate but more precise. Thus every measurement is approximate due to errors in measurement. In general, the errors in measurement can be broadly classified as (a) systematic errors and (b) random errors.
Table 2.5 Range and order of time intervals
Systematic errors
The systematic errors are those errors that
tend to be in one direction, either positive or negative. Some of the sources of systematic errors are :
(a) Instrumental errors that arise from the errors due to imperfect design or calibration of the measuring instrument, zero error in the instrument, etc. For example, the temperature graduations of a thermometer may be inadequately calibrated (it may read 104 °C at the boiling point of water at STP whereas it should read 100 °C); in a vernier callipers the zero mark of vernier scale may not coincide with the zero mark of the main scale, or simply an ordinary metre scale may be worn off at one end.
(b) Imperfection in experimental technique or procedure To determine the temperature of a human body, a thermometer placed under the armpit will always give a temperature lower than the actual value of the body temperature. Other external conditions (such as changes in temperature, humidity, wind velocity, etc.) during the experiment may systematically affect the measurement.
(c) Personal errors that arise due to an individual’s bias, lack of proper setting of the apparatus or individual’s carelessness in taking observations without observing proper precautions, etc. For example, if you, by habit, always hold your head a bit too far to the right while reading the position of a needle on the scale, you will introduce an error due to parallax.
Systematic errors can be minimised by improving experimental techniques, selecting better instruments and removing personal bias as far as possible. For a given set-up, these errors may be estimated to a certain extent and the necessary corrections may be applied to the readings.
Random errors
The random errors are those errors, which occur irregularly and hence are random with respect to sign and size. These can arise due to random and unpredictable fluctuations in experimental conditions (e.g. unpredictable fluctuations in temperature, voltage supply, mechanical vibrations of experimental set-ups, etc), personal (unbiased) errors by the observer taking readings, etc. For example, when the same person repeats the same observation, it is very likely that he may get different readings everytime.
Least count error
The smallest value that can be measured by the measuring instrument is called its least count. All the readings or measured values are good only up to this value.
The least count error is the error associated with the resolution of the instrument. For example, a vernier callipers has the least count as 0.01cm; a spherometer may have a least count of 0.001 cm. Least count error belongs to the category of random errors but within a limited size; it occurs with both systematic and random errors. If we use a metre scale for measurement of length, it may have graduations at 1 mm division scale spacing or interval.
Using instruments of higher precision, improving experimental techniques, etc., we can reduce the least count error. Repeating the observations several times and taking the arithmetic mean of all the observations, the mean value would be very close to the true value of the measured quantity.
2.6.1 Absolute Error, Relative Error and Percentage Error
(a) Suppose the values obtained in several measurements are a1, a2, a3...., an. The arithmetic mean of these values is taken as the best possible value of the quantity under the given conditions of measurement as :
amean = (a1+a2+a3+...+an ) / n (2.4)
or,
 (2.5)
(2.5)
This is because, as explained earlier, it is reasonable to suppose that individual measurements are as likely to overestimate as to underestimate the true value of the quantity.
The magnitude of the difference between the individual measurement and the true value of the quantity is called the absolute error of the measurement. This is denoted by |∆a |. In absence of any other method of knowing true value, we considered arithmatic mean as the true value. Then the errors in the individual measurement values from the true value, are
∆a1 = a1 – amean,
∆a2 = a2 – amean,
.... .... ....
.... .... ....
∆a n = an – amean
The ∆a calculated above may be positive in certain cases and negative in some other cases. But absolute error |∆a| will always be positive.
(b) The arithmetic mean of all the absolute errors is taken as the final or mean absolute error of the value of the physical quantity a. It is represented by ∆amean.
Thus,
∆amean = (|∆a1|+|∆a2 |+|∆a3|+...+ |∆an|)/n
(2.6)
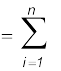 |∆ai|/n (2.7)
|∆ai|/n (2.7)
If we do a single measurement, the value we get may be in the range amean ± ∆amean
i.e. a = amean ± ∆amean
or,
amean – ∆amean ≤ a ≤ amean + ∆amean
(2.8)
This implies that any measurement of the physical quantity a is likely to lie between (amean+ ∆amean) and (amean− ∆amean).
(c) Instead of the absolute error, we often use the relative error or the percentage error (δa). The relative error is the ratio of the mean absolute error ∆amean to the mean value amean of the quantity measured.
Relative error = ∆amean/amean (2.9)
When the relative error is expressed in per cent, it is called the percentage error (δa).
Thus, Percentage error
δa = (∆amean/amean) × 100% (2.10)
Let us now consider an example.
Example 2.6 Two clocks are being tested against a standard clock located in a national laboratory. At 12:00:00 noon by the standard clock, the readings of the two clocks are :
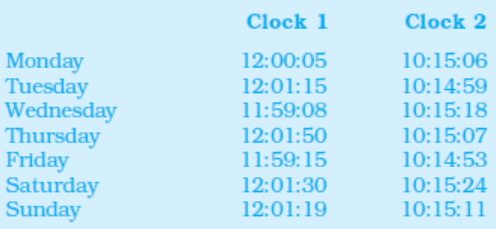
If you are doing an experiment that requires precision time interval measurements, which of the two clocks will you prefer ?
Answer The range of variation over the seven days of observations is 162 s for clock 1, and 31 s
