Table of Contents
Unit 6
General Principles and Processes of Isolation of Elements
Objectives
After studying this Unit, you will be able to:
- appreciate the contribution of Indian traditions in the metallurgical processes,
- explain the terms minerals, ores, concentration, benefaction, calcination, roasting, refining, etc.;
- understand the principles of oxidation and reduction as applied to the extraction procedures;
- apply the thermodynamic concepts like that of Gibbs energy and entropy to the principles of extraction of Al, Cu, Zn and Fe;
- explain why reduction of certain oxides like Cu2O is much easier than that of Fe2O3;
- explain why CO is a favourable reducing agent at certain temperatures while coke is better in some other cases;
- explain why specific reducing agents are used for the reduction purposes.
Thermodynamics illustrates why only a certain reducing element and a minimum specific temperature are suitable for reduction of a metal oxide to the metal in an extraction.
The history of civilisation is linked to the use of metals in antiquity in many ways. Different periods of early human civilisations have been named after metals. The skill of extraction of metals gave many metals and brought about several changes in the human society. It gave weapons, tools, ornaments, utensils, etc., and enriched the cultural life. The ‘Seven metals of antiquity’, as they are sometimes called, are gold, copper, silver, lead, tin, iron and mercury. Although modern metallurgy had exponential growth after Industrial Revolution, it is interesting to note that many modern concepts in metallurgy have their roots in ancient practices that pre-dated the Industrial Revolution. For over 7000 years, India has had a rich tradition of metallurgical skills.
The two important sources for the history of Indian metallurgy are archaeological excavations and literary evidences. The first evidence of metal in Indian subcontinent comes from Mehrgarh in Baluchistan, where a small copper bead, dated to about 6000 BCE was found. It is however thought to be native copper, which has not been extracted from the ore. Spectrometric studies on copper ore samples obtained from the ancient mine pits at Khetri in Rajasthan and on metal samples cut from representative Harappan artefacts recovered from Mitathal in Haryana and eight other sites distributed in Rajasthan, Gujarat, Madhya Pradesh and Maharashtra prove that copper metallurgy in India dates back to the Chalcolithic cultures in the subcontinent. Indian chalcolithic copper objects were in all probability made indigenously. The ore for extraction of metal for making the objects was obtained from chalcopyrite ore deposits in Aravalli Hills. Collection of archaeological texts from copper-plates and rock-inscriptions have been compiled and published by the Archaeological Survey of India during the past century. Royal records were engraved on copper plates (tamra-patra). Earliest known copper-plate has a Mauryan record that mentions famine relief efforts. It has one of the very few pre-Ashoka Brahmi inscriptions in India.
Harappans also used gold and silver, as well as their joint alloy electrum. Variety of ornaments such as pendants, bangles, beads and rings have been found in ceramic or bronze pots. Early gold and silver ornaments have been found from Indus Valley sites such as Mohenjodaro (3000 BCE). These are on display in the National Museum, New Delhi. India has the distinction of having the deepest ancient gold mines in the world, in the Maski region of Karnataka. Carbon dating places them in mid 1st millennium BCE.
Hymns of Rigveda give earliest indirect references to the alluvial placer gold deposits in India. The river Sindhu was an important source of gold in ancient times. It is interesting that the availability of alluvial placer gold in the river Sindhu has been reported in modern times also. It has been reported that there are great mines of gold in the region of Mansarovar and in Thokjalyug even now. The Pali text, Anguttara Nikaya narrates the process of the recovery of gold dust or particles from alluvial placer gold deposits. Although evidence of gold refining is available in Vedic texts, it is Kautilya’s Arthashastra, authored probably in 3rd or 4th century BCE, during Mauryan era, which has much data on prevailing chemical practices in a long section on mines and minerals including metal ores of gold, silver, copper, lead, tin and iron. Kautilya describes a variety of gold called rasviddha, which is naturally occurring gold solution. Kalidas also mentioned about such solutions. It is astonishing how people recognised such solutions.
The native gold has different colours depending upon the nature and amount of impurity present in it. It is likely that the different colours of native gold were a major driving force for the development of gold refining.
Recent excavations in central parts of Ganges Valley and Vindhya hills have shown that iron was produced there possibly as early as in 1800 BCE. In the recent excavations conducted by the Uttar Pradesh State Archaeological Department, iron furnaces, artefacts, tuyers and layers of slag have been found. Radiocarbon dating places them between BCE 1800 and 1000. The results of excavation indicate that the knowledge of iron smelting and manufacturing of iron artefacts was well known in Eastern Vindhyas and it was in use in the Central Ganga Plains, at least from the early 2nd millennium BCE. The quantity and types of iron artefacts and the level of technical advancements indicate that working of iron would have been introduced much earlier. The evidence indicates early use of iron in other areas of the country, which proves that India was indeed an independent centre for the development of the working of iron.
Iron smelting and the use of iron was especially established in South Indian megalithic cultures. The forging of wrought iron seems to have been at peak in India in the Ist millennium CE. Greek accounts report the manufacture of steel in India by crucible process. In this process, iron, charcoal and glass were mixed together in a crucible and heated until the iron melted and absorbed the carbon. India was a major innovator in the production of advanced quality steel. Indian steel was called ‘the Wonder Material of the Orient’. A Roman historian, Quintus Curtius, records that one of the gifts Porus of Taxila (326 BCE) gave to Alexander the Great was some two-and-a-half tons of Wootz steel. Wootz steel is primarily iron containing a high proportion of carbon (1.0 – 1.9%). Wootz is the English version of the word ‘ukku’ which is used for steel in Karnataka and Andhra Pradesh. Literary accounts suggest that Indian Wootz steel from southern part of the Indian subcontinent was exported to Europe, China and Arab world. It became prominent in the Middle East where it was named as Damasus Steel. Michael Faraday tried to duplicate this steel by alloying iron with a variety of metals, including noble metals, but failed.
When iron ore is reduced in solid state by using charcoal, porous iron blocks are formed. Therefore, reduced iron blocks are also called sponge iron blocks. Any useful product can be obtained from this material only after removing the porosity by hot forging. The iron so obtained is termed as wrought iron. An exciting example of wrought iron produced in ancient India is the world famous Iron Pillar. It was erected in its present position in Delhi in 5th century CE. The Sanskrit inscription engraved on it suggests that it was brought here from elsewhere during the Gupta Period. The average composition (weight%) of the components present in the wrought iron of the pillar, besides iron, are 0.15% C, 0.05% Si, 0.05% Mn, 0.25% P, 0.005% Ni, 0.03% Cu and 0.02% N. The most significant aspect of the pillar is that there is no sign of corrosion inspite of the fact that it has been exposed to the atmosphere for about 1,600 years.
Radiocarbon dating of charcoal from iron slag revealed evidence of continuous smelting in Khasi Hills of Meghalaya. The slag layer, which is dated to 353 BCE – CE 128, indicates that Khasi Hill region is the earliest iron smelting site studied in the entire region of North East India. The remnants of former iron-ore excavation and iron manufacturing are visible even now in the landscape of Khasi Hills. British naturalists who visited Meghalaya in early 19th century described the iron industry that had developed in the upper part of the Khasi Hills.
There is archaeological evidence of zinc production in Rajasthan mines at Zawar from the 6th or 5th BCE. India was the first country to master zinc distillation. Due to low boiling point, zinc tends to vapourise while its ore is smelted. Pure zinc could be produced after a sophisticated ‘downward’ distillation technique in which the vapour was condensed in a lower container. This technique was also applied to mercury. Indian metallurgists were masters in this technique. This has been described in Sanskrit texts of 14th century.
Indians had knowledge about mercury. They used it for medicinal purpose. Development of mining and metallurgy declined during the British colonial era. By the 19th century, once flourished mines of Rajasthan were mostly abandoned and became almost extinct. In 1947 when India got independence, European literature on science had already found its way slowly into the country. Thus, in post independence era, the Government of India initiated the process of nation building through the establishment of various institutes of science and technology. In the following sections, we will learn about the modern methods of extraction of elements.
6.1 Occurrence of Metals
A few elements like carbon, sulphur, gold and noble gases, occur in free state while others are found in combined forms in the earth’s crust. Elements vary in abundance. Among metals, aluminium is the most abundant. In fact, it is the third most abundant element in earth’s crust (8.3% approx. by weight). It is a major component of many igneous minerals including mica and clays. Many gemstones are impure forms of Al2O3. For example, gems ‘ruby’ and ‘sapphire’ have Cr and Co respectively as impurity. Iron is the second most abundant metal in the earth’s crust. It forms a variety of compounds and their various uses make it a very important element. It is one of the essential elements in biological systems as well.
For obtaining a particular metal, first we look for minerals which are naturally occurring chemical substances in the earth’s crust and are obtained through mining. Out of many minerals in which a metal may be found, only a few are viable to be used as source of that metal. Such minerals are known as ores.
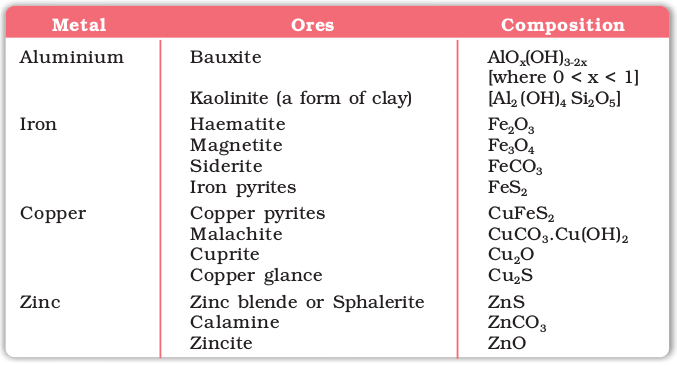
The principal ores of aluminium, iron, copper and zinc are given in Table 6.1.
Table 6.1: Principal Ores of Some Important Metals
A particular element may occur in a variety of compounds. The process of isolation of element from its compound should be such that it is chemically feasible and commercially viable.
For the purpose of extraction, bauxite is chosen for aluminium. For iron, usually the oxide ores which are abundant and do not produce polluting gases (like SO2 that is produced in case of iron pyrites) are taken. For copper and zinc, any of the ores listed in Table 6.1 may be used depending upon the availability and other relevant factors.
The entire scientific and technological process used for isolation of the metal from its ore is known as metallurgy. The extraction and isolation of an element from its combined form involves various principles of chemistry. Still, some general principles are common to all the extraction processes of metals.
An ore rarely contains only a desired substance. It is usually contaminated with earthly or undesired materials known as gangue. The extraction and isolation of metals from ores involves the following major steps:
• Concentration of the ore,
• Isolation of the metal from its concentrated ore, and
• Purification of the metal.
In the following Sections, we shall first describe the various steps for effective concentration of ores. After that principles of some of the common metallurgical processes will be discussed. Those principles will include the thermodynamic and electrochemical aspects involved in the effective reduction of the concentrated ore to the metal.
6.2 Concentration of Ores
Removal of the unwanted materials (e.g., sand, clays, etc.) from the ore is known as concentration, dressing or benefaction. Before proceeding for concentration, ores are graded and crushed to reasonable size. Concentration of ores involves several steps and selection of these steps depends upon the differences in physical properties of the compound of the metal present and that of the gangue. The type of the metal, the available facilities and the environmental factors are also taken into consideration. Some of the important procedures for concentration of ore are described below.
6.2.1 Hydraulic Washing
This is based on the difference between specific gravities of the ore and the gangue particles. It is therefore a type of gravity separation. In one such process, an upward stream of running water is used to wash the powdered ore. The lighter gangue particles are washed away and the heavier ore particles are left behind.
6.2.2 Magnetic Separation
This is based on differences in magnetic properties of the ore components. If either the ore or the gangue is attracted towards magnetic field, then the separation is carried out by this method. For example iron ores are attracted towards magnet, hence, non–magnetic impurities can be separted from them using magnetic separation. The powdered ore is dropped over a conveyer belt which moves over a magnetic roller (Fig.6.1) Magnetic substance remains attracted towards the belt and falls close to it.
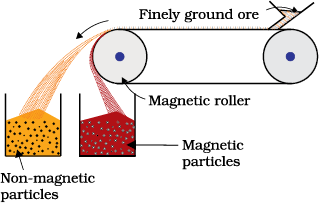
Fig. 6.1: Magnetic separation (schematic)
Froth Floatation Method
This method is used for removing gangue from sulphide ores. In this process, a suspension of the powdered ore is made with water. Collectors and froth stabilisers are added to it. Collectors (e.g., pine oils, fatty acids, xanthates, etc.) enhance non-wettability of the mineral particles and froth stabilisers (e.g., cresols, aniline) stabilise the froth.
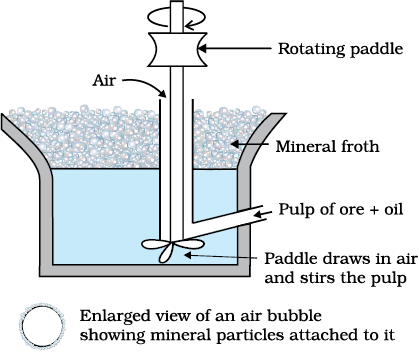
Fig. 6.2: Froth floatation process (schematic)
The mineral particles become wet by oils while the gangue particles by water. A rotating paddle agitates the mixture and draws air in it. As a result, froth is formed which carries the mineral particles. The froth is light and is skimmed off. It is then dried for recovery of the ore particles.
Sometimes, it is possible to separate two sulphide ores by adjusting proportion of oil to water or by using ‘depressants’. For example, in the case of an ore containing ZnS and PbS, the depressant used is NaCN. It selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
The Innovative Washerwoman
One can do wonders if he or she has a scientific temperament and is attentive to observations. A washerwoman had an innovative mind too. While washing a miner’s overalls, she noticed that sand and similar dirt fell to the bottom of the washtub. What was peculiar, the copper bearing compounds that had come to the clothes from the mines, were caught in the soapsuds and so they came to the top. One of her clients, Mrs. Carrie Everson was a chemist. The washerwoman told her experience to Mrs. Everson. The latter thought that the idea could be used for separating copper compounds from rocky and earth materials on large scale. This way an invention came up. At that time only those ores were used for extraction of copper, which contained large amounts of the metal. Invention of the Froth Floatation Method made copper mining profitable even from the low-grade ores. World production of copper soared and the metal became cheaper.
Leaching
Leaching is often used if the ore is soluble in some suitable solvent. Following examples illustrate the procedure:
(a) Leaching of alumina from bauxite
Bauxite is the principal ore of aluminium. It usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities. Concentration is carried out by heating the powdered ore with a concentrated solution of NaOH at 473 – 523 K and 35 – 36 bar pressure. This process is called digestion. This way, Al2O3 is extracted out as sodium aluminate. The impurity, SiO2 too dissolves forming sodium silicate. Other impurities are left behind.
Al2O3(s) + 2NaOH(aq) + 3H2O(l) → 2Na[Al(OH)4](aq) (6.1)
The sodium aluminate present in solution is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. At this stage, small amount of freshly prepared sample of hydrated Al2O3 is added to the solution. This is called seeding. It induces the precipitation.
2Na[Al(OH)4](aq) + 2CO2(g) → Al2O3.xH2O(s) + 2NaHCO3 (aq) (6.2)
Sodium silicate remains in the solution and hydrated alumina is filtered, dried and heated to give back pure Al2O3.
Al2O3.xH2O(s)![2310.png]() Al2O3(s) + xH2O(g) (6.3)
Al2O3(s) + xH2O(g) (6.3)
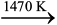 Al2O3(s) + xH2O(g) (6.3)
Al2O3(s) + xH2O(g) (6.3)(b) Other examples
In the metallurgy of silver and gold, the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air, which supplies O2. The metal is obtained later by replacement reaction.
4M(s) + 8CN–(aq)+ 2H2O(aq) + O2(g) → 4[M(CN)2]– (aq) +
4OH–(aq) (M= Ag or Au) (6.4)
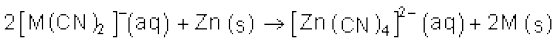 (6.5)
(6.5)Intext Questions
6.1 Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
6.2 What is the significance of leaching in the extraction of aluminium?
6.3 Extraction of Crude Metal from Concentrated Ore
To extract metal from concentrated ore, it must be converted to a form which is suitable for reduction to metal. Usually sulphide ores are converted to oxide before reduction because oxides are easier to reduce. Thus isolation of metals from concentrated ore involves two major steps viz.,
(a) conversion to oxide, and
(b) reduction of the oxide to metal.
(a) Conversion to oxide
(i) Calcination: Calcinaton involves heating. It removes the volatile matter which escapes leaving behind the metal oxide:
Fe2O3.xH2O(s) ![2330.png]() Fe2O3 (s) + xH2O(g) (6.6)
Fe2O3 (s) + xH2O(g) (6.6)
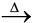 Fe2O3 (s) + xH2O(g) (6.6)
Fe2O3 (s) + xH2O(g) (6.6) ZnCO3 (s) ![2343.png]() ZnO(s) + CO2(g) (6.7)
ZnO(s) + CO2(g) (6.7)
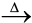 ZnO(s) + CO2(g) (6.7)
ZnO(s) + CO2(g) (6.7) CaCO3.MgCO3(s) ![2347.png]() CaO(s) + MgO(s ) + 2CO2(g) (6.8)
CaO(s) + MgO(s ) + 2CO2(g) (6.8)
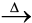 CaO(s) + MgO(s ) + 2CO2(g) (6.8)
CaO(s) + MgO(s ) + 2CO2(g) (6.8)(ii) Roasting: In roasting, the ore is heated in a regular supply of air in a furnace at a temperature below the melting point of the metal. Some of the reactions involving sulphide ores are:
2ZnS + 3O2 → 2ZnO + 2SO2 (6.9)
2PbS + 3O2 → 2PbO + 2SO2 (6.10)
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.11)
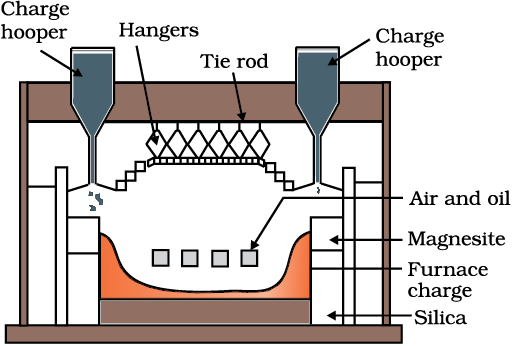
Fig. 6.3: A section of a modern reverberatory furnace
The sulphide ores of copper are heated in reverberatory furnace [Fig. 6.3]. If the ore contains iron, it is mixed with silica before heating. Iron oxide ‘slags of ’* as iron silicate and copper is produced in the form of copper matte which contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (6.12)
(slag)
The SO2 produced is utilised for manufacturing H2SO4 .
(b) Reduction of oxide to the metal
Reduction of the metal oxide usually involves heating it with a reducing agent, for example C, or CO or even another metal.
The reducing agent (e.g., carbon) combines with the oxygen of the metal oxide.
MxOy + yC → xM + y CO (6.13)
Some metal oxides get reduced easily while others are very difficult to be reduced (reduction means electron gain by the metal ion). In any case, heating is required.
6.4 Thermodynamic Principles of Metallurgy
Some basic concepts of thermodynamics help us in understanding the theory of metallurgical transformations. Gibbs energy is the most significant term. To understand the variation in the temperature required for thermal reductions and to predict which element will suit as the reducing agent for a given metal oxide (MxOy), Gibbs energy interpretations are made. The criterion for the feasibility of a thermal reduction is that at a given temperture Gibbs energy change of the reaction must be negative. The change in Gibbs energy, ∆G for any process at any specified temperature, is described by the equation:
∆G = ∆H – T∆S (6.14)
where, ∆H is the enthalpy change and ∆S is the entropy change for the process.
When the value of ∆G is negative in equation 6.14, only then the reaction will proceed. ∆G can become negative in the following situations:
1. If ∆S is positive, on increasing the temperature (T), the value of T∆S increases so that ∆H < T∆S. In this situation ∆G will become negative on increasing temperature.
2. If coupling of the two reactions, i.e. reduction and oxidation, results in negative value of ∆G for overall reaction, the final reaction becomes feasible. Such coupling is easily understood through Gibbs energy (∆rGV) vs T plots for the formation of the oxides (Fig. 6.4). These plots are drawn for free energy changes that occur when one gram mole of oxygen is consumed.
The graphical representation of Gibbs energy was first used by H.J.T. Ellingham. This provides a sound basis for considering the choice of reducing agent in the reduction of oxides. This is known as Ellingham Diagram. Such diagrams help us in predicting the feasibility of thermal reduction of an ore.
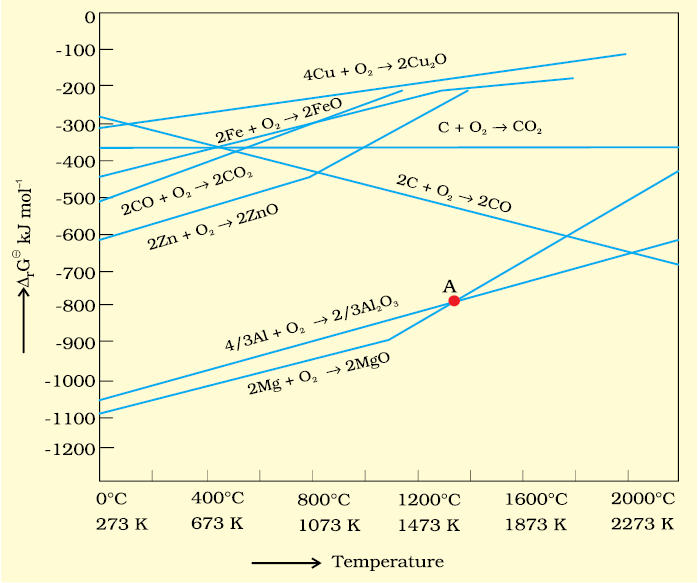
Fig. 6.4: Gibbs energy (∆rG0) vs T plots (schematic) for the formation of some oxides per mole of oxygen consumed (Ellingham diagram)
As we know, during reduction, the oxide of a metal decomposes and the reducing agent takes away the oxygen. The role of reducing agent is to provide ∆rGV negative and large enough to make the sum of ∆rGVof the two reactions, i.e, oxidation of the reducing agent and reduction of the metal oxide negative.
MxO(s) → xM (solid or liq) + ![2351.png]() O2 (g)
O2 (g) ![2356.png]() (6.15)
(6.15)
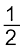 O2 (g)
O2 (g) 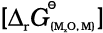 (6.15)
(6.15)If reduction is carried out by carbon the oxidation of the reducing agent (i.e., C) will be there:
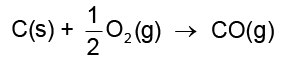
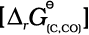 (6.16)
(6.16)There may also be complete oxidation of carbon to CO2.
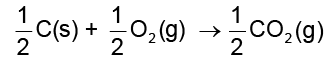
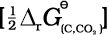 (6.17)
(6.17)On coupling (combing) reaction 6.15 and 6.16 we get:
MxO(s) + C(s) → xM(s or l) + CO(g) (6.18)
On coupling reaction 6.15 and 6.17 we have
MxO(s) + ![2404.png]() C(s) → xM(s or l) +
C(s) → xM(s or l) + ![2409.png]() CO2(g) (6.19)
CO2(g) (6.19)
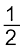 C(s) → xM(s or l) +
C(s) → xM(s or l) + 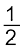 CO2(g) (6.19)
CO2(g) (6.19)Similarly, if carbon monoxide is reducing agent, reactions 6.15 and 6.20 given below need to be coupled.
CO(g) + ![2414.png]() O2(g) → CO2(g)
O2(g) → CO2(g) ![2419.png]() (6.20)
(6.20)
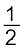 O2(g) → CO2(g)
O2(g) → CO2(g)  (6.20)
(6.20)Over all reaction will be as follows:
MxO(s) + CO(g) → xM(s or l) + CO2(g) (6.21)
Ellingham Diagram
(a) Ellingham diagram normally consists of plots of ∆fGV vs T for the formation of oxides of common metals and reducing agents i.e., for the reaction given below.
2xM(s) + O2(g) → 2MxO(s)
In this reaction, gas is consumed in the formation of oxide hence, molecular randomness decreases in the formation of oxide which leades to a negative value of ∆S as a result sign of T∆S term in equation (6.14) becomes positive. Subsequently ∆fGV shifts towards higher side despite rising T. The result is positive slope in the curve for most of the reactions for the formation of MxO(s).
(b) Each plot is a straight line and slopes upwards except when some change in phase
(s→ l or l→ g) takes place. The temperature at which such change occurs, is indicated by an increase in the slope on positive side (e.g., in the Zn, ZnO plot, the melting is indicated by an abrupt change in the curve) [Fig. 6.4].
(s→ l or l→ g) takes place. The temperature at which such change occurs, is indicated by an increase in the slope on positive side (e.g., in the Zn, ZnO plot, the melting is indicated by an abrupt change in the curve) [Fig. 6.4].
(c) When temperature is raised, a point is reached in the curve where it crosses ∆rGV=0 line. Below this temperature, ∆rGV for the formation of oxide is negative so MxO is stable. Above this point, free energy of formation of oxide is positive. The oxide, MxO will decompose on its own.
(d) Similar diagrams are constructed for sulfides and halides also. From them it becomes clear that why reduction of MxS is difficult.
Limitations of Ellingham Diagram
1. The graph simply indicates whether a reaction is possible or not, i.e., the tendency of reduction with a reducing agent is indicated. This is so because it is based only on the thermodynamic concepts. It does not explain the kinetics of the reduction process. It cannot answer questions like how fast reduction can proceed? However, it explains why the reactions are sluggish when every species is in solid state and smooth when the ore melts down. It is interesting to note here that ∆H (enthalpy change) and the ∆S(entropy change) values for any chemical reaction remain nearly constant even on varying temperature. So the only dominant variable in equation(6.14) becomes T. However, ∆S depends much on the physical state of the compound. Since entropy depends on disorder or randomness in the system, it will increase if a compound melts (s→ l) or vapourises
(l→ g) since molecular randomness increases on changing the phase from solid to liquid or from liquid to gas.
(l→ g) since molecular randomness increases on changing the phase from solid to liquid or from liquid to gas.
2. The interpretation of ∆rGV is based on K (∆GV = – RT lnK). Thus it is presumed that the reactants and products are in equilibrium:
MxO + Ared l xM + AredO
This is not always true because the reactant/product may be solid. In commercial processes
reactants and products are in contact for a short time.
reactants and products are in contact for a short time.
The reactions 6.18 and 6.21 describe the actual reduction of the metal oxide, MxO, that we want to accomplish. The ∆rG0 values for these reactions in general, can be obtained from the corresponding
∆f G0 values of oxides.
∆f G0 values of oxides.
As we have seen, heating (i.e., increasing T) favours a negative value of ∆rG0. Therefore, the temperature is chosen such that the sum of ∆rG0 in the two combined redox processes is negative. In ∆rG0 vs T plots (Ellingham diagram, Fig. 6.4), this is indicated by the point of intersection of the two curves, i.e, the curve for the formation of MxO and that for the formation of the oxide of the reducing substance. After that point, the ∆rG0 value becomes more negative for the combined process making the reduction of MxO possible. The difference in the two ∆rG0 values after that point determines whether reduction of the oxide of the element of the upper line is feasible by the element of which oxide formation is represented by the lower line. If the difference is large, the reduction is easier.
Example 6.1
Suggest a condition under which magnesium could reduce alumina.
Solution
The two equations are:
(a) ![2501.png]() Al + O2 →
Al + O2 → ![2506.png]() Al2O3 (b) 2Mg +O2 → 2MgO
Al2O3 (b) 2Mg +O2 → 2MgO
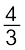 Al + O2 →
Al + O2 → 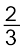 Al2O3 (b) 2Mg +O2 → 2MgO
Al2O3 (b) 2Mg +O2 → 2MgOAt the point of intersection of the Al2O3 and MgO curves (marked “A” in diagram 6.4), the ∆rG0 becomes ZERO for the reaction:
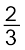 Al2O3 +2Mg → 2MgO +
Al2O3 +2Mg → 2MgO +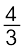 Al
AlBelow that point magnesium can reduce alumina.
Example 6.2
Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of aluminain the metallurgy of aluminium. Why ?
Solution
Temperatures below the point of intersection of Al2O3 and MgO curves, magnesium can reduce alumina. But the process will be uneconomical.
Example 6.3
Why is the reduction of a metal oxide easier if the metal formed is in liquid state at the temperature of reduction?
Solution
The entropy is higher if the metal is in liquid state than when it is in solid state. The value of entropy change (∆S) of the reduction process is more on positive side when the metal formed is in liquid state and the metal oxide being reduced is in solid state. Thus the value of ∆rGV becomes more on negative side and the reduction becomes easier.
(a) Extraction of iron from its oxides
After concentration, mixture of oxide ores of iron (Fe2O3, Fe3O4) is subjected to calcination/roasting to remove water, to decompose carbonates and to oxidise sulphides. After that these are mixed with limestone and coke and fed into a Blast furnace from its top, in which the oxide is reduced to the metal. In the Blast furnace, [Fig. 6.5] reduction of iron oxides takes place at different temperature ranges. A blast of hot air is blown from the bottom of the furnace by burning coke in the lower portion to give temperature upto about 2200K. The burning of coke, therefore, supplies most of the heat required in the process. The CO and heat move to the upper part of the furnace. In upper part, the temperature is lower and the iron oxides (Fe2O3 and Fe3O4) coming from the top are reduced in steps to FeO. These reactions can be summarised as follows:
At 500 – 800 K (lower temperature range in the blast furnace),
Fe2O3 is first reduced to Fe3O4 and then
to FeO
to FeO
3 Fe2O3 + CO → 2 Fe3O4 + CO2 (6.22)
Fe3O4 + 4 CO → 3Fe + 4 CO2 (6.23)
Fe2O3 + CO → 2FeO + CO2 (6.24)
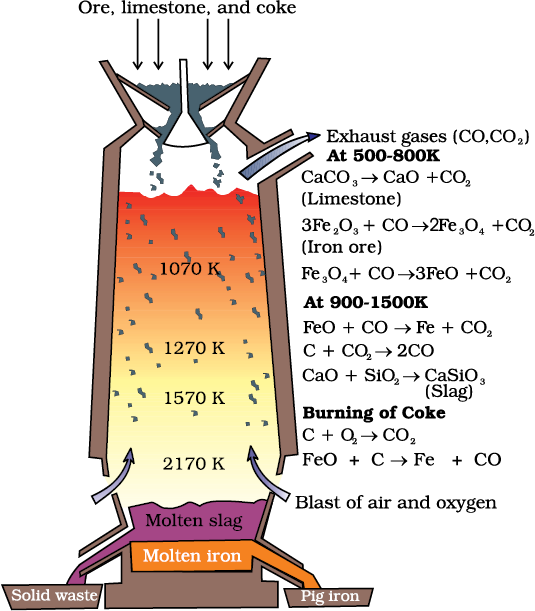
Fig. 6.5: Blast furnace
Limestone is also decomposed to CaO which removes silicate impurity of the ore as slag. The slag is in molten state and separates out from iron.
At 900 – 1500 K (higher temperature range in the blast furnace):
C + CO2 → 2 CO (6.25)
FeO + CO → Fe + CO2 (6.26)
Thermodynamics helps us to understand how coke reduces the oxide and why this furnace is chosen. One of the main reduction steps in this process involves reaction 6.27 given below.
FeO(s) + C(s) → Fe(s/l) + CO (g) (6.27)
This reaction can be seen as a reaction in which two simpler reactions have coupled. In one the reduction of FeO is taking place and in the other, C is being oxidised to CO:
FeO(s) → Fe(s) + ![2431.png]() O2(g)
O2(g) ![2436.png]() (6.28)
(6.28)
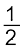 O2(g)
O2(g) 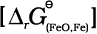 (6.28)
(6.28) C(s) + ![2447.png]() O2 (g) → CO (g)
O2 (g) → CO (g) ![2452.png]() (6.29)
(6.29)
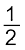 O2 (g) → CO (g)
O2 (g) → CO (g) 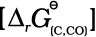 (6.29)
(6.29)When both the reactions take place to yield the equation (6.27), the net Gibbs energy change becomes:
∆rG0 (C, CO) + ∆rG0 (FeO, Fe) = ∆rG0 (6.30)
Naturally, the resultant reaction will take place when the right hand side in equation 6.30 is negative. In ∆rG0 vs T plot representing the change Fe→ FeO in Fig. 6.6 goes upward and that representing the change C→ CO (C,CO) goes downward. They cross each other at about 1073K. At temperatures above 1073K (approx.), the C, CO line is below the Fe, FeO line ![2458.png]() <
< ![2463.png]() . So above 1073 K in the range of temprature 900–1500 K coke will reduce FeO and will itself be oxidised to CO. Let us try to understand this through Fig. 6.6 (approximate values of ∆rG0 are given). At about 1673K (1400oC) ∆rG0 value for the reaction:
. So above 1073 K in the range of temprature 900–1500 K coke will reduce FeO and will itself be oxidised to CO. Let us try to understand this through Fig. 6.6 (approximate values of ∆rG0 are given). At about 1673K (1400oC) ∆rG0 value for the reaction:
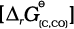 <
< 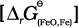 . So above 1073 K in the range of temprature 900–1500 K coke will reduce FeO and will itself be oxidised to CO. Let us try to understand this through Fig. 6.6 (approximate values of ∆rG0 are given). At about 1673K (1400oC) ∆rG0 value for the reaction:
. So above 1073 K in the range of temprature 900–1500 K coke will reduce FeO and will itself be oxidised to CO. Let us try to understand this through Fig. 6.6 (approximate values of ∆rG0 are given). At about 1673K (1400oC) ∆rG0 value for the reaction: 2FeO→ 2Fe+O2 is +341 kJmol-1 because it is reverse of Fe→ FeO change and for the reaction
2C+O2→ 2CO ∆rG0 is -447 kJmol-1. If we calculate ∆rG0 value for overall reaction (6.27 the value will be -53 kJmol-1). Therefore, reaction 6.27 becomes feasible. In a similar way the reduction of Fe3O4 and Fe2O3 by CO at relatively lower temperatures can be explained on the basis of lower lying points of intersection of their curves with the CO, CO2 curve.
The iron obtained from Blast furnace contains about 4% carbon and many impurities in smaller amount (e.g., S, P, Si, Mn). This is known as pig iron. It can be moulded into variety of shapes. Cast iron is different from pig iron and is made by melting pig iron with scrap iron and coke using hot air blast. It has slightly lower carbon content (about 3%) and is extremely hard and brittle.
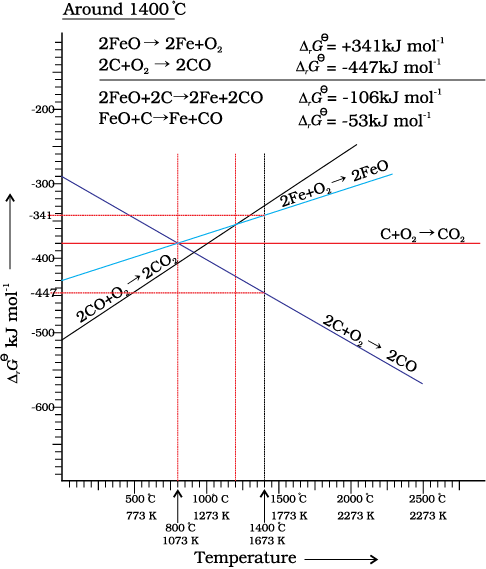
Fig. 6.6: Gibbs energy Vs T plot (schematic) for the formation of oxides of iron and carbon (Ellingham diagram)
Further Reductions
Wrought iron or malleable iron is the purest form of commercial iron and is prepared from cast iron by oxidising impurities in a reverberatory furnace lined with haematite. The haematite oxidises carbon to carbon monoxide:
Fe2O3 + 3 C → 2 Fe + 3 CO (6.31)
Limestone is added as a flux and sulphur, silicon and phosphorus are oxidised and passed into the slag. The metal is removed and freed from the slag by passing through rollers.
(b) Extraction of copper from cuprous oxide [copper(I) oxide]
In the graph of ∆rG0 vs T for the formation of oxides (Fig. 6.4), the Cu2O line is almost at the top. So it is quite easy to reduce oxide ores of copper directly to the metal by heating with coke. The lines (C, CO) and
(C, CO2) are at much lower positions in the graph particularly after
500 – 600K. However, many of the ores are sulphides and some may also contain iron. The sulphide ores are roasted/smelted to give oxides:
(C, CO2) are at much lower positions in the graph particularly after
500 – 600K. However, many of the ores are sulphides and some may also contain iron. The sulphide ores are roasted/smelted to give oxides:
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.32)
The oxide can then be easily reduced to metallic copper using coke:
Cu2O + C → 2 Cu + CO (6.33)
In actual process, the ore is heated in a reverberatory furnace after mixing with silica. In the furnace, iron oxide ‘slags of’ as iron slicate is formed. Copper is produced in the form of copper matte. This contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (6.34)
(Slag)
Copper matte is then charged into silica lined convertor. Some silica is also added and hot air blast is blown to convert the remaining FeS, FeO and Cu2S/Cu2O to the metallic copper. Following reactions take place:
2FeS + 3O2 → 2FeO + 2SO2 (6.35)
FeO + SiO2 → FeSiO3 (6.36)
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.37)
2Cu2O + Cu2S → 6Cu + SO2 (6.38)
The solidified copper obtained has blistered appearance due to the evolution of SO2 and so it is called blister copper.
(c) Extraction of zinc from zinc oxide
The reduction of zinc oxide is done using coke. The temperature in this case is higher than that in the case of copper. For the purpose of heating, the oxide is made into brickettes with coke and clay.
ZnO + C ![2467.png]() Zn + CO (6.39)
Zn + CO (6.39)
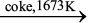 Zn + CO (6.39)
Zn + CO (6.39)The metal is distilled off and collected by rapid chilling.
Intext Questions
6.3 The reaction,
Cr2O3+2Al → Al2O3+2Cr (∆rG0= – 421kJ)
is thermodynamically feasible as is apparent from the Gibbs energy value.
Why does it not take place at room temperature?
Why does it not take place at room temperature?
6.4 Is it true that under certain conditions, Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
6.5 Electrochemical Principles of Metallurgy
We have seen how principles of thermodyamics are applied to pyrometallurgy. Similar principles are effective in the reductions of metal ions in solution or molten state. Here they are reduced by electrolysis or by adding some reducing element.
In the reduction of a molten metal salt, electrolysis is done. Such methods are based on electrochemical principles which could be understood through the equation,
∆G0 = – nE0F (6.40)
here n is the number of electrons and E0 is the electrode potential of the redox couple formed in the system. More reactive metals have large negative values of the electrode potential. So their reduction is difficult.
If the difference of two E0 values corresponds to a positive E0 and consequently negative ∆G0 in equation 6.40, then the less reactive metal will come out of the solution and the more reactive metal will go into the solution, e.g.,
If the difference of two E0 values corresponds to a positive E0 and consequently negative ∆G0 in equation 6.40, then the less reactive metal will come out of the solution and the more reactive metal will go into the solution, e.g.,
Cu2+ (aq) + Fe(s) → Cu(s) + Fe2+ (aq) (6.41)
In simple electrolysis, the Mn+ ions are discharged at negative electrodes (cathodes) and deposited there. Precautions are taken considering the reactivity of the metal produced and suitable materials are used as electrodes. Sometimes a flux is added for making the molten mass more conducting.
Aluminium
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AlF6 or CaF2 which lowers the melting point of the mixture and brings conductivity. The fused matrix is electrolysed. Steel vessel with lining of carbon acts as cathode and graphite anode is used. The overall reaction may be written as:
2Al2O3 + 3C → 4Al + 3CO2 (6.42)
This process of electrolysis is widely known as Hall-Heroult process.
Thus electrolysis of the molten mass is carried out in an electrolytic cell using carbon electrodes. The oxygen liberated at anode reacts with the carbon of anode producing CO and CO2. This way for each kg of aluminium produced, about 0.5 kg of carbon anode is burnt away. The electrolytic reactions are:
Cathode: Al3+ (melt) + 3e– → Al(l) (6.43)
Anode: C(s) + O2– (melt) → CO(g) + 2e– (6.44)
C(s) + 2O2– (melt) → CO2 (g) + 4e– (6.45)
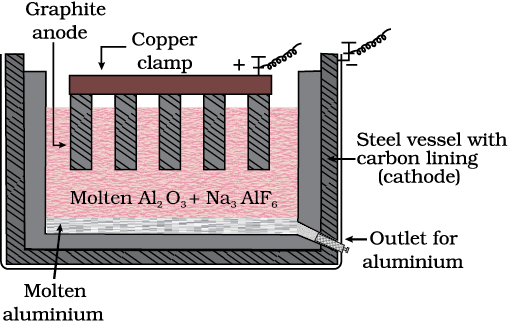
Fig. 6.7: Electrolytic cell for the extraction of aluminium
Copper from Low Grade Ores and Scraps
Copper is extracted by hydrometallurgy from low grade ores. It is leached out using acid or bacteria. The solution containing Cu2+ is treated with scrap iron or H2 (equations 6.40; 6.46).
Cu2+(aq) + H2(g) → Cu(s) + 2H+ (aq) (6.46)
Example 6.4
At a site, low grade copper ores are available and zinc and iron scraps are also available. Which of the two scraps would be more suitable for reducing the leached copper ore and why?
Solution
Zinc being above iron in the electrochemical series (more reactive metal is zinc), the reduction will be faster in case zinc scraps are used. But zinc is costlier metal than iron so using iron scraps will be advisable and advantageous.
6.6 Oxidation Reduction
Besides reductions, some extractions are based on oxidation particularly for non-metals. A very common example of extraction based on oxidation is the extraction of chlorine from brine (chlorine is abundant in sea water as common salt) .
2Cl–(aq) + 2H2O(l) → 2OH–(aq) + H2(g) + Cl2(g) (6.47)
The ∆G0 for this reaction is + 422 kJ. When it is converted to E0 (using ∆G0 = – nE0F), we get E0 = – 2.2 V. Naturally, it will require an external emf that is greater than 2.2 V. But the electrolysis requires an excess potential to overcome some other hindering reactions (Unit–3, Section 3.5.1). Thus, Cl2 is obtained by electrolysis giving out H2 and aqueous NaOH as by-products. Electrolysis of molten NaCl is also carried out. But in that case, Na metal is produced and not NaOH.
As studied earlier, extraction of gold and silver involves leaching the metal with CN–. This is also an oxidation reaction (Ag → Ag+ or Au → Au+). The metal is later recovered by displacement method.
4Au(s) + 8CN–(aq) + 2H2O(aq) + O2(g) →
4[Au(CN)2]–(aq) + 4OH–(aq) (6.48)
2[Au(CN)2]–(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2– (aq) (6.49)
In this reaction zinc acts as a reducing agent.
6.7 Refining
A metal extracted by any method is usually contaminated with some impurity. For obtaining metals of high purity, several techniques are used depending upon the differences in properties of the metal and the impurity. Some of them are listed below.
(a) Distillation
(b) Liquation
(c) Electrolysis
(d) Zone refining
(e) Vapour phase refining
(f) Chromatographic methods
These are described in detail here.
(a) Distillation
This is very useful for low boiling metals like zinc and mercury. The impure metal is evaporated to obtain the pure metal as distillate.
(b) Liquation
In this method a low melting metal like tin can be made to flow on a sloping surface. In this way it is separated from higher melting impurities.
(c) Electrolytic refining
In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the same metal. The more basic metal remains in the solution and the less basic ones go to the anode mud. This process is also explained using the concept of electrode potential, over potential, and Gibbs energy which you have seen in previous sections. The reactions are:
Anode: M → Mn+ + ne–
Cathode: Mn+ + ne– → M (6.50)
Copper is refined using an electrolytic method. Anodes are of impure copper and pure copper strips are taken as cathode. The electrolyte is acidified solution of copper sulphate and the net result of electrolysis is the transfer of copper in pure form from the anode to the cathode:
Anode: Cu → Cu2+ + 2 e–
Cathode: Cu2+ + 2e– → Cu (6.51)
Impurities from the blister copper deposit as anode mud which contains antimony, selenium, tellurium, silver, gold and platinum; recovery of these elements may meet the cost of refining. Zinc may also be refined this way.
(d) Zone refining
This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. A mobile heater surrounding the rod of impure metal is fixed at its one end (Fig. 6.8). The molten zone moves along with the heater which is moved forward. As the heater moves forward, the pure metal crystallises out of the melt left behind and the impurities pass on into the adjacent new molten zone created by movement of heaters. The process is repeated several times and the heater is moved in the same direction again and again. Impurities get concentrated at one end. This end is cut off. This method is very useful for producing semiconductor and other metals of very high purity, e.g., germanium, silicon, boron, gallium and indium.
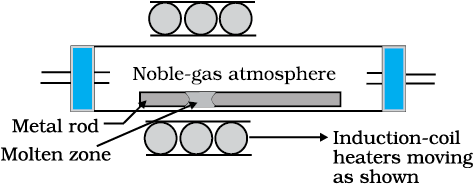
Fig. 6.8: Zone refining process
(e) Vapour phase refining
In this method, the metal is converted into its volatile compound which is collected and decomposed to give pure metal. So, the two requirements are:
(i) the metal should form a volatile compound with an available reagent,
(ii) the volatile compound should be easily decomposable, so that the recovery is easy.
Following examples will illustrate this technique.
Mond Process for Refining Nickel: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex named as nickel tetracarbonyl. This compex is decomposed at higher temperature to obtain pure metal.
Ni + 4CO ![2478.png]() Ni(CO)4 (6.52)
Ni(CO)4 (6.52)
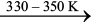 Ni(CO)4 (6.52)
Ni(CO)4 (6.52) Ni(CO)4 ![2490.png]() Ni + 4CO (6.53)
Ni + 4CO (6.53)
 Ni + 4CO (6.53)
Ni + 4CO (6.53)van Arkel Method for Refining Zirconium or Titanium: This method is very useful for removing all the oxygen and nitrogen present in the form of impurity in certain metals like Zr and Ti. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilises:
Zr + 2I2 → ZrI4 (6.54)
The metal iodide is decomposed on a tungsten filament, electrically heated to about 1800K. The pure metal deposits on the filament.
ZrI4 → Zr + 2I2 (6.55)
(f) Chromatographic methods
You have learnt about chromatographic technique of purification of substances in Class XI (Unit–12).
Column chromatography is very useful for purification of the elements which are available in minute quantities and the impurities are not very different in chemical properties from the element to be purified.
Aluminium foils are used as wrappers for food materials. The fine dust of the metal is used in paints and lacquers. Aluminium, being highly reactive, is also used in the extraction of chromium and manganese from their oxides. Wires of aluminium are used as electricity conductors. Alloys containing aluminium, being light, are very useful.
6.8 Uses of Aluminium, Copper, , Zinc and Iron
Copper is used for making wires used in electrical industry and for water and steam pipes. It is also used in several alloys that are rather tougher than the metal itself, e.g., brass (with zinc), bronze (with tin) and coinage alloy (with nickel).
Zinc is used for galvanising iron. It is also used in large quantities in batteries. It is constituent of many alloys, e.g., brass, (Cu 60%, Zn 40%) and german silver (Cu 25-30%, Zn 25-30%, Ni 40–50%). Zinc dust is used as a reducing agent in the manufacture of dye-stuffs, paints, etc.
Cast iron, which is the most important form of iron, is used for casting stoves, railway sleepers, gutter pipes , toys, etc. It is used in the manufacture of wrought iron and steel. Wrought iron is used in making anchors, wires, bolts, chains and agricultural implements. Steel finds a number of uses. Alloy steel is obtained when other metals are added to it. Nickel steel is used for making cables, automobiles and aeroplane parts, pendulum, measuring tapes. Chrome steel is used for cutting tools and crushing machines, and stainless steel is used for cycles, automobiles, utensils, pens, etc.
Summary
Although modern metallurgy had exponential growth after Industrial Revolution, many modern concepts in metallurgy have their roots in ancient practices that predated the Industrial Revolution. For over 7000 years, India has had high tradition of metallurigical skills. Ancient Indian metallurgists have made major contributions which deserve their place in metallurgical history of the world. In the case of zinc and high–carbon steel, ancient India contributed significantly for the developemnt of base for the modern metallurgical advancements which induced metallurgical study leading to Industrial Revolution.
Metals are required for a variety of purposes. For this, we need their extraction from the minerals in which they are present and from which their extraction is commercially feasible.These minerals are known as ores. Ores of the metal are associated with many impurities. Removal of these impurities to certain extent is achieved in concentration steps. The concentrated ore is then treated chemically for obtaining the metal. Usually the metal compounds (e.g., oxides, sulphides) are reduced to the metal. The reducing agents used are carbon, CO or even some metals. In these reduction processes, the thermodynamic and electrochemical concepts are given due consideration. The metal oxide reacts with a reducing agent; the oxide is reduced to the metal and the reducing agent is oxidised. In the two reactions, the net Gibbs energy change is negative, which becomes more negative on raising the temperature. Conversion of the physical states from solid to liquid or to gas, and formation of gaseous states favours decrease in the Gibbs energy for the entire system. This concept is graphically displayed in plots of ∆G0 vs T (Ellingham diagram) for such oxidation/reduction reactions at different temperatures. The concept of electrode potential is useful in the isolation of metals (e.g., Al, Ag, Au) where the sum of the two redox couples is positive so that the Gibbs energy change is negative. The metals obtained by usual methods still contain minor impurities. Getting pure metals requires refining. Refining process depends upon the differences in properties of the metal and the impurities. Extraction of aluminium is usually carried out from its bauxite ore by leaching it with NaOH. Sodium aluminate, thus formed, is separated and then neutralised to give back the hydrated oxide, which is then electrolysed using cryolite as a flux. Extraction of iron is done by reduction of its oxide ore in blast furnace. Copper is extracted by smelting and heating in a reverberatory furnace. Extraction of zinc from zinc oxides is done using coke. Several methods are employed in refining the metal. Metals, in general, are very widely used and have contributed significantly in the development of a variety of industries.
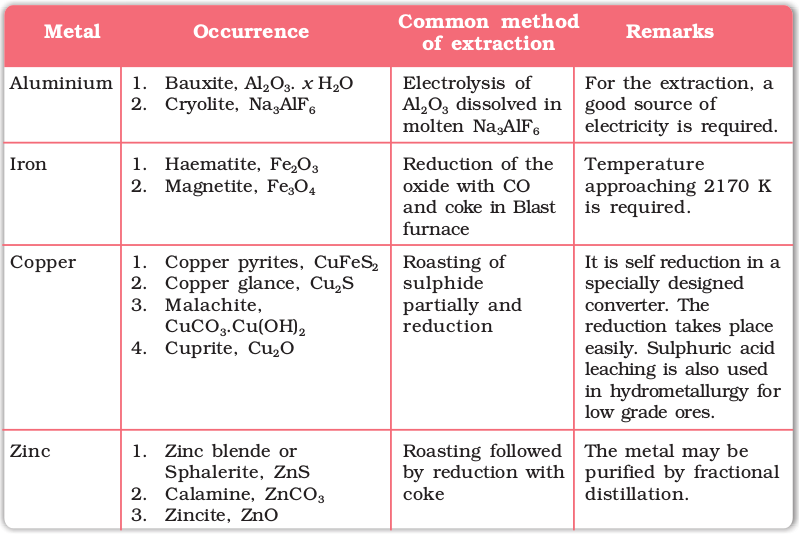
A Summary of the Occurrence and Extraction of some Metals is Presented in the following Table
Exercises
6.1 Copper can be extracted by hydrometallurgy but not zinc. Explain.
6.2 What is the role of depressant in froth floatation process?
6.3 Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
6.4 Explain: (i) Zone refining (ii) Column chromatography.
6.5 Out of C and CO, which is a better reducing agent at 673 K ?
6.6 Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
6.7 Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
6.8 Write chemical reactions taking place in the extraction of zinc from zinc blende.
6.9 State the role of silica in the metallurgy of copper.
6.10 Which method of refining may be more suitable if element is obtained in minute quantity?
6.11 Which method of refining will you suggest for an element in which impurities present have chemical properties close to the properties of that elements?
6.12 Describe a method for refining nickel.
6.13 How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
6.14 Giving examples, differentiate between ‘roasting’ and ‘calcination’.
6.15 How is ‘cast iron’ different from ‘pig iron”?
6.16 Differentiate between “minerals” and “ores”.
6.17 Why copper matte is put in silica lined converter?
6.18 What is the role of cryolite in the metallurgy of aluminium?
6.19 How is leaching carried out in case of low grade copper ores?
6.20 Why is zinc not extracted from zinc oxide through reduction using CO?
6.21 The value of ∆fG0 for formation of Cr2 O3 is – 540 kJmol−1and that of Al2 O3 is – 827 kJmol−1. Is the reduction of Cr2 O3 possible with Al ?
6.22 Out of C and CO, which is a better reducing agent for ZnO ?
6.23 The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
6.24 Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
6.25 What is the role of graphite rod in the electrometallurgy of aluminium?
6.26 Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
6.27 Predict conditions under which Al might be expected to reduce MgO.
(Hint: See Intext question 6.4)
Answers to Some Intext Questions
6.1 Ores in which one of the components (either the impurity or the actual ore) is magnetic can be concentrated, e.g., ores containing iron (haematite, magnetite, siderite and iron pyrites).
6.2 Leaching is significant as it helps in removing the impurities like SiO2, Fe2O3, etc. from the bauxite ore.
6.3 Certain amount of activation energy is essential even for such reactions which are thermodynamically feasible, therefore heating is required.
6.4 Yes, below 1350°C Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO. This can be inferred from ∆GV Vs T plots (Fig. 6.4).
