With the help of a labelled diagram, describe the method of separating ammonium chloride from a mixture of ammonium chloride and common salt. Mention the difference in the properties of ammonium chloride and sodium chloride which has made this separations possible.
There are many substances which are converted into gas from solid when heated, and converted from gas to solid when cooled without converting into liquid. Such substances are known as sublime. For example – ammonium chloride, naphthalene balls, camphor, etc. Therefore, mixture of one sublime and other substance can be separated using the method of sublimation.
The mixture of ammonium chloride and common salt can be separated out using the process of sublimation. For this, the mixture is heated in a China dish. The China dish is covered by an inverted funnel. Cotton is used for plugging the opening of the funnel. After heating, ammonium chloride is converted into vapour and gets deposited over the inner surface of funnel; due to cooling. This leaves the common salt in China dish. Ammonium chloride can be taken out by scratching from the inner wall of funnel.
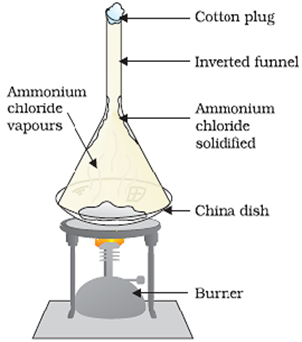
The process of sublimation is used to separate that component of a solid-solid mixture which sublimes on heating. Hence the property of ammonium chloride to sublime has made this separations possible.