A strip of impure copper metal is given to you. Describe briefly how you will purify it by using the chemical effect of electric current. Draw a labelled diagram of the experimental set up used for this purpose.
Copper ion is positively charged. It will be attracted towards the plate which is connected to the negative terminal of the battery. Because positive charges attract negative charges and repel positive charges.
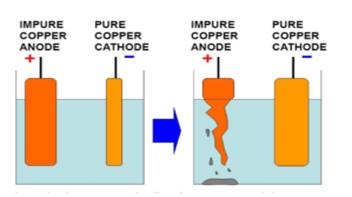
As copper ions from the impure copper electrode are transferred to the thin copper plate, this thin pure copper plate must be connected to the negative terminal of the battery. Consequently, impure copper rod is connected to the positive terminal of the battery. In this way, pure copper is deposited on the negative electrode i.e. cathode.