The rms speed of oxygen at room temperature is about 500 m/s. The rms speed of hydrogen at the same temperature is about
Root mean square velocity of gas is given as
vrms =![]()
where R=gas constant whose value is 8.31 J/mol K
T=temperature of gas
M= molar mass of molecule of gas
rms speed of oxygen = ![]() …(i)
…(i)
rms speed of hydrogen=![]() …(ii)
…(ii)
Given:
vrms(o)=500m/s
and we know molar mass of oxygen =M(o)=32
molar mass of hydrogen=M(H)=2
Diving equation (i) and (ii) we get
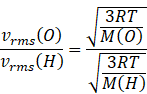
Since speed of both gases must be calculated at same temperature this equation will reduce to
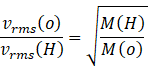
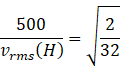
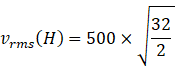
![]() m/s (
m/s (![]() )
)
![]() Root mean square velocity of hydrogen is 2000m/s.
Root mean square velocity of hydrogen is 2000m/s.
1