The process on ideal gas, shown in figure is
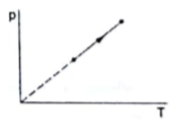
From the graph, we can conclude that pressure is directly proportional to temperature because it is straight line graph between pressure and temperature.
We know that ideal gas equation is
PV=nRT
Where V= volume of gas
R=gas constant
T=temperature
n=number of moles of gas
P=pressure of gas.
![]()
From above equation, we can see that pressure will be directly proportional to temperature only when volume V is also a constant.
So, only for isochoric process (a process where volume is kept constant) pressure will be directly proportional to temperature.
1