Consider a mixture of oxygen and hydrogen kept at room temperature. As compared to a hydrogen molecule an oxygen molecule hits the wall
In kinetic theory of ideal gas, the average energy is given by
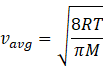
Where R=gas constant=8.31Jmol-1K-1
T=temperature of gas
M=molar mass of gas
1. From the formula for average speed, it can be seen that ![]() .
.
2. Molar mass of hydrogen molecule is 2 amu and molar mass of oxygen molecule is 16 amu.
3. So, molar mass of oxygen molecule is greater than molar mass of hydrogen molecule.
4. Therefore, average speed of oxygen molecule will be less than average speed of hydrogen molecule.
1