The temperature and pressure at Shimla are 15.0°C and 72.0 cm of mercury and at Kalka these are 35.0°C and 76.0 cm of mercury. Find the ratio of air density at Kalka to the air density at Shimla.
We know ideal gas equation
PV=nRT
Where V= volume of gas
R=gas constant
T=temperature
n=number of moles of gas
P=pressure of gas
Number of moles n =![]() =
=![]()
Density ![]()
Given:
Temperature of Shimla T1=15.0°C
T(K)=T (![]() )+273.15
)+273.15
T1=T(K)=15+273.15=288.15K
Pressure of Shimla P1 = 72.0 cm of mercury
Temperature of Kalka T2 = 35.0°C
T(K)=T (![]() )+273.15
)+273.15
T2=T(K)=35+273.15=308.15K
Pressure of Kalka P2=76.0 cm of mercury
Substituting the value of n and ![]() in ideal gas equation, we get
in ideal gas equation, we get
![]() =
=![]()
So,
![]()
![]()
![]()
Taking the ratio of equations (i) and (ii),
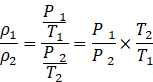
![]()
![]()
![]() The ratio of air density at Kalka to the air density at Shimla is 0.98.
The ratio of air density at Kalka to the air density at Shimla is 0.98.