A sample of 0.177 g of an ideal gas occupies 1000 cm3 at STP. Calculate the rms speed of the gas molecules.
STP means standard temperature-273.15K and pressure 101.325 kPa.
We know that rms speed of gas is given by
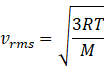
Where R=gas constant 8.31J/molK
T=temperature of gas
M=molar mass of gas
Given
Temperature T=273.15K
Pressure P=101.325![]() Pa
Pa
Mass =0.177g =0.177![]() 10-3kg
10-3kg
Volume = 1000cm3
1cm=10-2m
1cm3=10-6m3
1000cm3=10-3m3
Density ![]() =
=![]()
We know that ideal gas equation is
PV=nRT
Where V= volume of gas
R=gas constant=8.31J/molK
T=temperature
N=number of moles of gas
So, we can write ![]() .
.
Putting the value of RT in vrms we get
vrms =![]()
nM= total mass of gas ‘m’
and density ![]() =
=![]()
Putting the value of density ![]() and m in vrms, we get
and m in vrms, we get
vrms =![]()
Putting the value of P and ![]() in above equation we get
in above equation we get
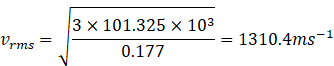
![]() The rms speed of the gas molecules at STP is 1310.4ms-1.
The rms speed of the gas molecules at STP is 1310.4ms-1.