The mean speed of the molecules of a hydrogen sample equals the mean speed of the molecules of a helium sample. Calculate the ratio of the temperature of the hydrogen sample to the temperature of the helium sample.
In kinetic theory of ideal gas mean speed also known as average
speed is given as
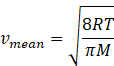
Where R=gas constant=8.31Jmol-1K-1
T=temperature of gas
M=molar mass of gas
Given
![]()
Let temperature of hydrogen gas =T(H)
Temperature of helium gas= T(He)
Molar mass of hydrogen gas =2amu
Molar mass of helium gas =4amu
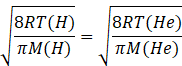
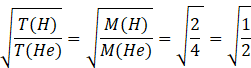
Squaring both sides
![]()
the ratio of the temperature of the hydrogen sample to the temperature of the helium sample is 1:2.
1