At what temperature the mean sped of the molecules of hydrogen gas equals the escape speed from the earth?
In kinetic theory of ideal gas mean speed also known as average speed is given as
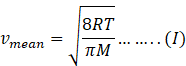
Where R=gas constant=8.31Jmol-1K-1
T-temperature of gas
M=molar mass of gas
Molar mass of hydrogen=2amu=2g/mol=2![]() kg/mol
kg/mol
Escape speed of earth is the speed given to projectile so that it escapes the gravitational field of earth. It is given by formula
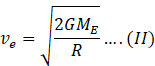
Where G=universal gravitational constant
ME=mass of earth
R=radius of earth
We also know that acceleration due to gravity g is
![]()
Multiplying and dividing equation (II) by R
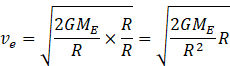
Putting the value of g from equation (III) to above equation we get
![]()
According to question vmean is equal to ve
So, from equation (I) and (IV) we get
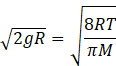
Radius of earth R=6400km=6400000m
g=9.8m/s
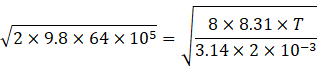
Squaring both sides
![]()
![]()
Temperature at which the mean sped of the molecules of hydrogen gas equals the escape speed from the earth is 11800K.