Find the ratio of the mean speed of hydrogen molecules to the mean speed of nitrogen molecules in a sample containing a mixture of the two gases.
In kinetic theory of ideal gas, mean speed also known as average
speed is given as
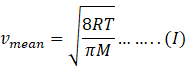
Where R=gas constant=8.31Jmol-1K-1
T=temperature of gas
M=molar mass of gas
Molar mass of hydrogen molecule M(H)=2 amu
Molar mass of nitrogen molecule M(N)=28 amu
Mean speed of hydrogen molecule=
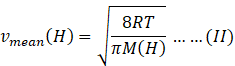
Mean speed of nitrogen molecule=
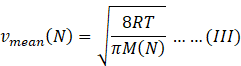
Temperature of both the gases is same.
Dividing equation (II) and (III) we get
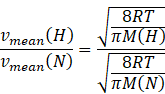
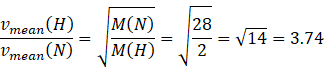
![]() The ratio of the mean speed of hydrogen molecules to the mean speed of nitrogen molecules in a sample containing a mixture of the two gases is 3.74.
The ratio of the mean speed of hydrogen molecules to the mean speed of nitrogen molecules in a sample containing a mixture of the two gases is 3.74.
1