Hydrogen gas is contained in a closed vessel at 1 atm (100 kPa) and 300 K
(a) Calculate the means speed of the molecules.
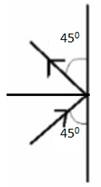
(b) Suppose the molecules strike the wall with this speed making an average angle of 45° with it.
How many molecules strike each square meter of the wall per second?
(a)
In kinetic theory of ideal gas, mean speed also known as average speed is given as
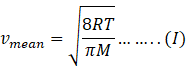
Where R=gas constant=8.31Jmol-1K-1
T=temperature of gas
M=molar mass of gas
Given
T=300k
Molar mass of hydrogen gas =2amu=2g/mol=2![]() kg/mol
kg/mol
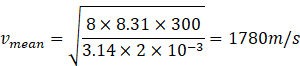
(b)
Let velocity be u=![]() from part (a)
from part (a)
From figure we can see that
Total Momentum in vertical direction
mucos45![]() -mucos45
-mucos45![]() =0
=0
Total momentum in horizontal direction
musin45![]() -(-musin45
-(-musin45![]() )=2musin45
)=2musin45![]() =2mu
=2mu![]() mu
mu
Total change momentum of 1 molecules =![]() mu
mu
Total change momentum of n molecules =![]() nmu
nmu
We know that,
![]()
Let ‘t’ be the time taken to changing the momentum.
So, force per unit second due to 1 molecule=![]() mu
mu
Force per unit second due to n molecule=![]() nmu
nmu
Given
Pressure by n molecule=105 Pa
Area=1m2
Pressure =![]()
![]()
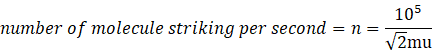
We know that
Mass of 6.023![]() of hydrogen molecule=2
of hydrogen molecule=2![]() kg
kg
Mass of 1 hydrogen molecule m =![]() kg
kg
Therefore
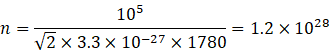
![]() Number of molecules strike each square meter of the wall per second=1.2
Number of molecules strike each square meter of the wall per second=1.2![]()