During an experiment, an ideal gas is found to obey an additional law pV2 = constant. The gas is initially at a temperature T and volume V. Find the temperature when it expands to a volume 2V.
We know ideal gas equation
PV=nRT
Where V= volume of gas
R=gas constant=8.31Jmol-1K-1
T=temperature
n=number of moles of gas
P=pressure of gas
So ![]() ………. (I)
………. (I)
Now differentiating the ideal gas equation, we get
PdV + VdP=nRdT …………. (II) (we have applied product rule for
differentiation of PV)
Now as given in question the ideal here follows and additional law which is PV2=constant.
So, differentiating this additional law as well we get
2PVdV + V2dP=0
Taking V as common we get
2PdV + VdP=0 ……… (III)
Subtract equation (III) from (II)
2PdV + VdP - PdV - VdP = -nRdT
PdV=-nRdT
From equation (I), substitute the value of P in above equation we get
![]()
![]()
Integrating equation (IV) from limits V to 2V and T1 to T2
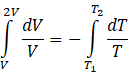
We know ![]() . Applying this formula
. Applying this formula
![]()
![]()
Where we have applied the property of ln which is
ln(a)-ln(b)=ln(a/b)
![]()
![]()
So, the temperature at which the gas expands is half of the initially temperature.