A container of volume 50 cc contains air (mean molecular weight = 28.8 g) and is open to atmosphere where the pressure is 100 kPa. The container is kept in a bath containing melting ice (0°C).
(a) Find the mass of the air in the container when thermal equilibrium is reached.
(b) The container is now placed in another bath containing boiling water (100°C).
Find the mass of air in the container.
(c) The container is now closed and placed in the melting-ice bath. Find the pressure of the air when thermal equilibrium is reached.
Given
Volume of container V1=50cc=50![]() 10-6m3
10-6m3
Molecular mass of air in container M= 28.8g
Pressure of air P1= 100kPa=105Pa
(a) We know ideal gas equation
PV=nRT
Where V= volume of gas
R=gas constant=8.31Jmol-1K-1
T=temperature
n=number of moles of gas
P=pressure of gas
In first case the air is kept in container having ice. So, temperature in case will be T1 =0![]() =273.15K
=273.15K
Number of moles n=![]() …. (1)
…. (1)
Number of moles n=![]() …. (2)
…. (2)
Equating (1) and (2) we get
![]()
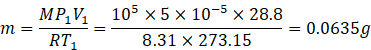
So, mass of air when temperature is 0![]() is 0.0635g.
is 0.0635g.
(b) Now in second case the container having air is kept in a bath having boiling water. So, temperature will be T2=100![]() =373.15K.
=373.15K.
Since, now temperature is 100![]() therefore, some of the air will be expelled as air will expand but the volume of container is fixed. So, some of the air will go out of the container as container is open.
therefore, some of the air will be expelled as air will expand but the volume of container is fixed. So, some of the air will go out of the container as container is open.
So, first we will calculate the mass of air expelled from container and then we will subtract it from the original volume V1 to get the mass of remaining air.
Pressure will be same as before, as the air is still open to atmosphere. So P2=P1.
Let the volume of expanded gas be V2. Number of moles in volume V2 be the same as before because no extra gas is added. It has just expanded.
![]()
As P2=P1, therefore
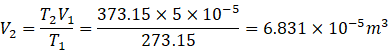
Volume of gas expelled out the container
V =V2-V1=![]()
Number of moles of expelled gas
![]()
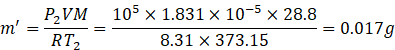
So, the mass of gas remaining in the container
=m’-m=0.0635-0.017=0.0465g
So, the mass of gas when temperature is 100![]() is 0.0456g.
is 0.0456g.
(c) Now the container is kept in ice bath i.e. temperature 0![]() and container is closed. So, now the pressure will change.
and container is closed. So, now the pressure will change.
Number of moles left =![]()
Applying ideal gas equation
![]()
![]() Pressure of gas when lid is closed, and temperature is 0
Pressure of gas when lid is closed, and temperature is 0![]() is 73.1kPa.
is 73.1kPa.