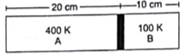
Figure shows a cylindrical tube of length 30 cm which is partitioned by a tight-fitting separator. The separator is very weakly conducting and can freely slide along the tube. Ideal gases are filled in the two parts of the vessel. In the beginning, the temperatures in the parts A and B are 400 K and 100 K respectively. The separator slides to a momentary equilibrium position shown in the figure. Find the final equilibrium position of the separator, reached after a long time.
Let the initial pressure of the chamber A and B be Pa and Pb respectively.
Let the final pressure of the chamber A and B be P’a and P’b respectively.
Let the curved surface area of tube be A
Given:
Length of chamber A=20cm=0.2m
Length of chamber B=10cm=0.1m
Volume =area![]() height
height
Initial volume of chamber A=Va=0.2A
Initial volume of chamber B=Vb=0.1A
Initial Temperature of chamber A=Ta=400K
Initial Temperature of chamber B=Tb=100K
For first (momentary) equilibrium, pressure of both chamber will be same.
Pa=Pb
Let the final temperature at equilibrium be T.
We know that ideal gas equation
PV=nRT
Where V= volume of gas
R=gas constant =8.3JK-1mol-1
T=temperature
n=number of moles of gas
P=pressure of gas.
Then,
For chamber A, number of moles, before and after final equilibrium will be same as no new gas has been added. So, applying ideal gas equation, before and after final equilibrium and equation nR, we get,
![]()
![]()
Similarly, for chamber B
![]()
![]()
At second equilibrium pressures on both sides will be same again.
P’a=P’b
![]()
![]()
Now Pa=Pb so,
V’b =2![]() V’a ……(iii)
V’a ……(iii)
Volume of chamber A plus volume of chamber B will be equal to total volume. So,
V’b+V’a=V=0.3A
2V’a+V’a=0.3A (from (iii))
3V’a=0.3A
V’a=0.1A
Now we know that volume=length![]() area. So,
area. So,
V’a=lA
Where l=length of chamber A after equilibrium
lA=0.1A
l=0.1m=10cm
![]() length of chamber A after equilibrium is 10 cm.
length of chamber A after equilibrium is 10 cm.