One mole of an ideal gas undergoes a process
![]()
where p0 and V0 are constants. Find the temperature of the gas when V = V0.
Given
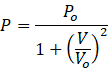
Multiplying both sides by V
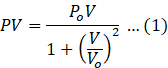
We know ideal gas equation
PV=nRT
Where V= volume of gas
R=gas constant=8.31Jmol-1K-1
T=temperature
n=number of moles of gas
P=pressure of gas
Here, it is given that number of moles n=1
So, PV=RT. Putting this value of PV in equation 1
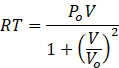
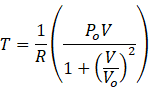
According to question V=Vo
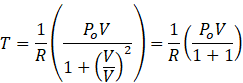
![]()
![]() The temperature of the gas when V = V0 is
The temperature of the gas when V = V0 is ![]() .
.
1