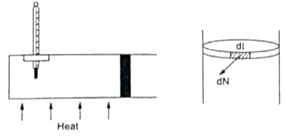
Figure shows a cylindrical tube of radius 5 cm and length 20 cm. It is closed by a tight-fitting cork. The friction coefficient between the cork and the tube is 0.20. The tube contains an ideal gas at a pressure of 1 atm and a temperature of 300 K. The tube is slowly heated, and it is found that the cork pops out when the temperature reaches 600 K. Let dN denote the magnitude of the normal contact force exerted by a small length dl of the cork along the periphery (see the figure). Assuming that the temperature of the gas is uniform at any instant, calculate dN/dl.
Given
Pressure of gas P1=1atm=105Pa
Radius of tube R=5cm=0.05m
So, area of tube = ![]() (0.05)2
(0.05)2
Length of tube =20cm=0.2m
So, volume=area![]() length
length
Volume of cylindrical tube = ![]() (0.05)2
(0.05)2![]() 0.2=0.0016m2
0.2=0.0016m2
Initial temperature T1 =300K
Final temperature T2=600K
Coefficient of friction ![]() =0.2
=0.2
Let final pressure be P2. So, volume of the gas remains same until pressure becomes P2 and then corks pop out. Number of moles will also be same. So, we can apply ideal gas equation which is
PV=nRT
Where V= volume of gas
R=gas constant =8.3JK-1mol-1
T=temperature
n=number of moles of gas
P=pressure of gas.
![]()
![]()
![]()
Net pressure on cork = P2-P1=2![]() 105 - 105=105Pa
105 - 105=105Pa
We know that
![]()
So, force acting on the cork=pressure on cork![]() area of cork
area of cork
F=105![]() (0.05)2
(0.05)2
According to law of friction,
F=![]() N
N
Where F=force of friction
N=normal to the surface of cork
![]() =coffieicent of friction
=coffieicent of friction
Now, friction is always equal to the applied force until body starts to slide.
So,
![]()
In question N is denoted as dN. So, N=dN
And dl=length of cork around periphery of cork i.e. dl=circumference of cork.
![]()
Thus, the value of ![]() .
.