Figure shows a cylindrical tube of cross-sectional area A fitted with two frictionless pistons. The pistons are connected to each other by a metallic wire. Initially, the temperature of the gas is T0 and its pressure is p0 which equals the atmospheric pressure.
(a) What is the tension in the wire?
(b) What will be the tension if the temperature is increased to 2T0?
![]()
(a) Initially, the pressure inside the cylinder is equal to atmospheric pressure as given in question. So, pressure (thus force) on piston will be same from outside the cylinder and inside the cylinder which is atmospheric pressure. Therefore, no net force will act on piston and tension in wire will be zero.
(b)
Given
Initial temperature T1=To
Increased temperature T2=2To
Initial pressure of gas P1=Po=105Pa
Let curved surface area of cylinder be A
Let the piston be L distance apart
Volume =area![]() length=A
length=A![]() L
L
Since, no new gas is added to cylinder so, number of moles, before and after temperature change will be same and the volume of cylinder is same. So, applying ideal gas equation, before and after the increase of temperature.
PV=nRT
Where V= volume of gas
R=gas constant =8.3JK-1mol-1
T=temperature
n=number of moles of gas
P=pressure of gas.
![]()
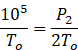
P2=2![]() 105=2P1=2Po
105=2P1=2Po
Net pressure P2-P1=2Po-Po=Po
Net force acting outwards is force=pressure![]() area
area
F=Po![]() A ……(i)
A ……(i)
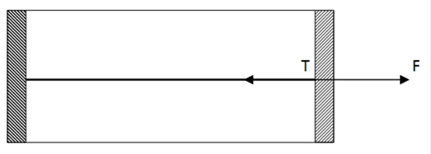
Since, temperature is increased, gas inside the cylinder will expand and piston will move outward freely without any acceleration, as the piston is frictionless. So, from diagram F>T
From newton second law of motion, which states that ‘net external force on particle is equal to rate of change of momentum’, we can write
F-T=0
Rate of change of momentum will be zero because piston moves without any acceleration. So, ![]()
Therefore,
F=T= Po![]() A ( from (i))
A ( from (i))
![]() The tension if the temperature is increased to 2T0 is PoA.
The tension if the temperature is increased to 2T0 is PoA.