The human body has an average temperature of 98°F. Assume that the vapor pressure of the blood in the veins behaves like that of pure water. Find the minimum atmospheric pressure which is necessary to prevent the blood from boiling. Use figure of the text for the vapor pressures.
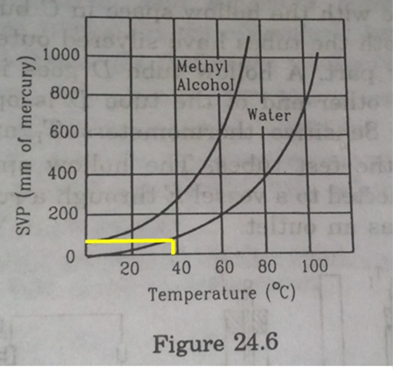
Minimum atmospheric pressure necessary to prevent blood from boiling is 50mm of Hg.
Explanation
Given
Average temperature of human body=98![]()
T (![]() )=98
)=98![]()
T (![]() )=
)=![]()
Now above 36.7![]() , the human blood will start boiling. So, the minimum atmospheric pressure to prevent boiling of human blood will the pressure corresponding to this temperature.
, the human blood will start boiling. So, the minimum atmospheric pressure to prevent boiling of human blood will the pressure corresponding to this temperature.
So, from figure pressure corresponding to 36.7![]() temperature on y-axis is 50mm of Hg which shown by yellow line in the figure.
temperature on y-axis is 50mm of Hg which shown by yellow line in the figure.
1