Figure shows two process A and B on a system. Let ΔQ1 and ΔQ2 be the heat given to the system in processes A and B respectively. Then
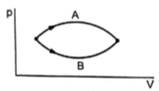
Initial and final points of both processes A and B are same. Therefore, internal energy in both the processes will be the same because internal energy is a state variable, independent of the path taken.
The area under the P-V curve gives the work done on the system. From the graph, it can be seen the area under the curve for process A is more than the area under the curve for process B., therefore, work done on the system in process A ΔW1 is more than work done on the system in process B ΔW2
ΔW1 > ΔW2 …..(i)
According to First law of thermodynamics,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied/extracted to/from the system
ΔU=change in internal energy
ΔW=work done by/on the system
For process A
ΔQ1=ΔU+ΔW1 …(ii)
For process B
ΔQ2=ΔU+ΔW2 …(iii)
From equation (i) ,(ii) and (iii) it is clear that ΔQ1 > ΔQ2.