An ideal gas goes from the state i to the state f as shown in the figure. The work done by the gas during the process.
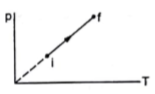
Since the graph between P and T is a straight line passing through the origin, therefore P![]() T.
T.
P can only be proportional to T when the volume is kept constant. This can be easily proved from the ideal gas equation which is
PV=RT
Since R is already a constant, if volume also becomes constant then P![]() T.
T.
Constant volume implies ΔV=0.
We know that,
Work done = force ×displacement
![]()
Volume = area ×displacement
Therefore,
Work done=pressure ×volume
Let change in the volume of system = ΔV = V2-V1
Pressure =P
Thus, work done by the system W
W=PΔV
For ΔV=0, W=0.
1