Figure shows three paths through which a gas can be taken from the state A to the state B. Calculate the work done by the gas in each of the three paths.
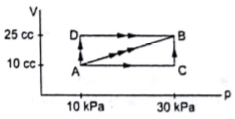
We know that work done by the gas is given as
ΔW=PΔV
From graph we can write
VA =VC = 10cc=10×10-6m3
VD=VB = 25cc= 25×10-6m3
PB=PC=30kPa= 30×103Pa
PA=PD=10kPa=10×103Pa
Work done in path ADB WADB=WAD + WDB
WADB = PA (VD-VA) + 0 (∵ WDB =0 because VD=VB)
=10×103×(25-10)×10-6
=0.15J
Work done in path AB WAB= Pavg(VB-VA)
![]()
WAB=20×103× (25-10)×10-6
=0.30J
Work done in path ACB WACB= WAC+WBC
WACB = 0+PB(VB-VC) (∵ WAC=0 because VA=VC)
=30×103×(25-10)×10-6
=0.45J
1