When a system is taken through the process abc shown in figure 80J of heat is absorbed by the system and 30 J of work is done by it. If the system does 10 J of work during the process adc, how much heat flows into it during the process?
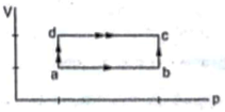
Given
Heat absorbed in process abc ΔQ1=80J
Work done by the system in process abc ΔW1=30J
Work done by the system in process adc ΔW2=10J
Let heat absorbed into the system during process adc =ΔQ2
Now initial point a and final point c is the same for both the processes is the same. So, change in internal energy will be the same for both the process, as internal energy is a state function independent of the path taken.
Therefore,
ΔU1=ΔU2=ΔU ……(i)
From first law of thermodynamics, we know that,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied to the system
ΔU=change in internal energy
ΔW=work done by the system
Using first law of thermodynamics for process abc
ΔQ1=ΔU1+ΔW1
ΔU1=ΔQ1-ΔW1=80-30=50J
Using first law of thermodynamics for process adc
ΔQ2=ΔU2+ΔW2
=ΔU1+ΔW2 (from (i))
= 50+10=60J
∴ heat absorbed into the system during process adc= 60J.