Calculate the heat absorbed by a system in going through the cyclic process shown in the figure.
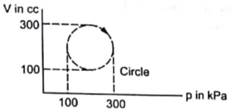
We know that in the cyclic process the system returns to its initial state. So, change internal energy in the cyclic process will be zero as internal energy is a state function.
From first law of thermodynamics, we know that,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied to the system
ΔU=change in internal energy
ΔW=work done by the system
Since ΔU=0, first law becomes
ΔQ=ΔW
In a PV graph work done is equal to the area under the curve.
ΔW= area of a circle
ΔQ=ΔW=area of circle
Diameter of the circle =300-100=200
![]()
Area of the circle =π × (radius)2
=π × 100×100×10-6×103
(10-6×103 is because volume and pressure are given in cc and kPa respectively)
Area of circle =3.14×10=31.4
∴ Heat absorbed by a system = 31.4J.