A substance is taken through the process abc as shown in the figure. If the internal energy of the substance increases by 5000 J and heat of 2625 cal is given to the system, calculate the value of J.
‘J' is mechanical equivalent of heat a conversion factor between two different units of energy: calorie to the joule.
Given
Heat given to system =2625cal =2625×J J
Change in internal energy= 5000J
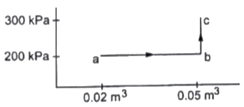
From graph
Va=0.02m3
Vb=Vc=0.05m3
Pa=Pb=200kPa=200×103Pa
Pc=300kPa=300×103Pa
We know that work done by the gas is given as
ΔW=PΔV
Work done in process abc=ΔW=Wab+Wbc
ΔW=Pa(Vb-Va)+0 (∵ Wbc=0 because Vb=Vc)
ΔW=200×103×(0.05-0.02)=6000J
From first law of thermodynamics, we know that,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied to the system
ΔU=change in internal energy
ΔW=work done by the system
ΔQ=5000+6000=11000J
But ΔQ=2625cal =2625×J J
Therefore,
2625×J=11000
![]()
∴ value of ‘J’ is 4.19joule/cal.