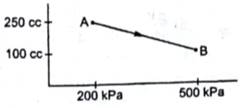
A gas is taken along the path AB as shown in the figure. If 70 cal of heat is extracted from the gas in the process, calculate the change in the internal energy of the system.
Given
Heat extracted from the system ΔQ=-70cal=-70×4.2=-294J
The negative sign is because heat is extracted from the system.
From graph
VA=250cc=250×10-6m3
VB=100cc=100×10-6m3
PA=200kPa=200×103Pa
PB=500kPa=500×103Pa
We know that work done by the gas is given as
ΔW=PΔV
Here since we have two values of pressure we will take average pressure.
![]()
ΔW=Pavg(VA-VB)
= 350×103× (250-100) ×10-6
=-52.5J
From first law of thermodynamics, we know that,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied to the system
ΔU=change in internal energy
ΔW=work done by the system
Therefore,
ΔU=-294-(-52.5) =-241.5J
∴ change in internal energy is -241.5J.