Consider the cyclic process ABCA, shown in the figure, performed on a sample 2.0 mol of an ideal gas. A total of 1200 J of heat is withdrawn from the sample in the process. Find the work done by the gas during the part BC.
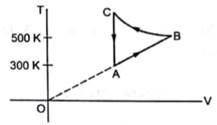
Given
Heat extracted from the system ΔQ=-1200J
(negative sign is because heat is extracted from the system)
Number of moles of the gas n=2.0
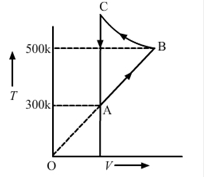
From the graph we can write
TA=300K
TB=500K
VA=VC
We know that work done by the gas is given as
ΔW=PΔV
Where ΔV =change in volume
P =pressure
So, work done along line CA will be zero, as VA=VC.
Thus, total work done will be
ΔW=WAB+WBC
WAB = P(VB-VA)
But we know that ideal gas equation is
PV=nRT
Where n= number of moles
R=gas constant
T=temperature
∴ PΔV=nRΔT
Thus, we can write WAB = P(VB-VA) = nR(TB-TA)
∴ ΔW= nR(TB-TA) + WBC
From first law of thermodynamics, we know that,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied to the system
ΔU=change in internal energy
ΔW=work done by the system
Process ABCA is a cyclic process. The system is brought back to its initial state. Since internal energy is a state function, change in internal energy will be zero.
So, ΔU=0.
So, first law becomes
ΔQ=ΔW
ΔQ= nR(TB-TA) + WBC
We know that R=8.31J/K mol
WBC =ΔQ- nR(TB-TA)
= -1200 - 2×8.31× (500-300)
=-1200 – 3324
=-4524J
Thus, work done along path BC =-4524J