Figure shows a cylindrical tube of volume V with adiabatic walls containing an ideal gas. The internal energy of this ideal gas is given by 1.5 nRT. The tube is divided into two equal parts by a fixed diathermic wall. Initially, the pressure and the temperature are p1, T1 on the left and p2, T2 on the right. The system is left for sufficient time so that the temperature becomes equal on the two sides.
(a) How much work has been done by the gas on the left part?
(b) Find the final pressures on the two sides.
(c) Find the final equilibrium temperature.
(d) How much heat has flown from the gas on the right to the gas on the left?
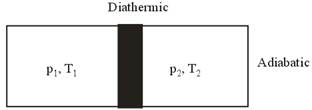
a) According to the question, the diathermic separator between both the part is fixed. So, no change in volume will be observed. And as we know work done on gas is P![]() V. Therefore, no work will be done on the left part during the process as the volume is not changing.
V. Therefore, no work will be done on the left part during the process as the volume is not changing.
First, we will calculate the final temperature and then final pressure.
(c) Given
Pressure of left chamber =P1
Pressure of right chamber =P2
Temperature of left chamber =T1
Temperature of right chamber =T2
Let the number of moles in the left chamber be n1
Number of moles in the right chamber be n2
Diathermic wall has divided the tube in two equal part. So, the volume of the left and the right chamber will be V/2.
Applying ideal gas equation in the left chamber,
![]()
![]()
Similarly applying ideal gas equation in the right chamber,
![]()
![]()
Total number of moles n=n1 +n2
![]()
![]()
![]()
The internal energy of ideal gas is given as
U=nCvT
Where Cv=molar specific heat at constant volume
T=temperature.
According to question,
U=1.5nRT
![]() nCvT=1.5nRT
nCvT=1.5nRT
So, Cv=1.5R
The internal energy of the left chamber U1=n1CvT1
The internal energy of right chamber U2= n2CvT2
Total internal energy U= U1+U2
1.5nRT= n1CvT1+ n2CvT2
1.5nRT=Cv(n1T1+n2T2)
Substituting the value of Cv
1.5nRT=1.5R(n1T1+n2T2)
nT=n1T1+n2T2
substituting the value of n1 and n2 in above equation
![]()
![]()
![]()
Substituting the value of n in the above equation,
![]()
![]()
Thus, final equilibrium temperature T=![]() .
.
(b) Now we will find final pressures on both sides.
Let final pressure of left chamber P1’
Final pressure of right chamber P2’
Applying ideal gas equation in the left chamber before and after equilibrium
![]()
![]()
From equation (i) and (ii),
![]()
![]()
Substituting the value of T,
![]()
Applying ideal gas equation in the right chamber before and after equilibrium
![]()
![]()
From equation (iii) and (iv),
![]()
![]()
Substituting the value of T,
![]()
(d) The internal energy of ideal gas is given as
U=nCvT
Where Cv=molar specific heat at constant volume
T=temperature.
As stated in part (a) no work will be done on either chamber of the vessel as the diathermic separator is fixed.
So, ΔW=0 for the right chamber of the tube.
From first law of thermodynamics, we know that,
ΔQ=ΔU+ΔW
Where ΔQ=heat supplied to the system
ΔU=change in internal energy
ΔW=work done by the system
ΔQ=ΔU
Change in internal energy of the right chamber after equilibrium has reached will be
ΔU=n2CvT2 -n2CvT
Substituting the value of n2, Cv and T in the above equation
![]()
![]()
![]()
![]()
![]()
![]()
Thus, heat flown from left to right chamber is![]() .
.