Figure shows water in a container having 2.0 mm thick walls made of a material of thermal conductivity 0.50 W m–1 °C–1. The container is kept in a melting ice bath at 0°C. The total surface area in contact with water is 0.05 m2. A wheel is clamped inside the water and is coupled to a block of mass M as shown in the figure. As the block goes down, the wheel rotates. It is found that after some time a steady state is reached in which the block goes down with a constant speed of 10 cm s–1 and the temperature of the water remains constant at 1.0°C. Find the mass M of the block. Assume that the heat flows out of the water only through the walls in contact. Take g = 10 m s–2.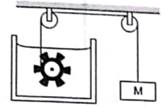
Given:
Thickness of the container: x = 2 mm = 0.002 m
Thermal conductivity of the container: K= 0.50 W m–1 °C–1.
Temperature of the water: T1 = 1 °C
Temperature of the ice bath: T2 = 0 °C
Surface are in contact with the water: 0.05 m2
Speed of the block: v = 10 cm/s = 0.1 m/s
g = 10 m/s2
Formula used:
Rate of amount of heat flowing or heat current is given as:![]()
Here, Δθ is the amount of heat transferred, ΔT is the temperature difference, K is the thermal conductivity of the material, A is the area of cross section of the material and x is the thickness or length of the material.
The effect of the block going down and the heat transfer has one identity in common, which is power.
Power due to a block of mass M moving with constant velocity is given as![]()
![]()
Here W is the work done by the block.
∴ P = (mg).v
∴ P = M× 10× 0.1
∴ P = M× 1
Also in terms of Heat Energy: P= Energy per unit time![]()
![]()
![]()
![]()
Hence, the mass of the block is 12.5 kg.