On a winter day when the atmospheric temperature drops to –10°C, ice forms on the surface of a lake.
(a) Calculate the rate of increase of thickness of the ice when 10 cm of ice is already formed.
(b) Calculate the total time taken in forming 10 cm of ice. Assume that the temperature of the entire water reaches 0°C before the ice starts forming. Density of water = 1000 kg m–3, latent heat of fusion of ice = 3.36 × 105 J kg–1 and thermal conductivity of ice = 1.7 W m–1 °C–1. Neglect the expansion of water on freezing.
Given:
Temperature of the water: T1 = 0 °C
Temperature of the atmosphere: T2 = -10 °C
Change in temperature : ΔT = T1-T2 = 10 °C
Length of the ice formed : l = 10 cm = 0.1 m
Density of water: ρ = 1000 kg m–3
Latent heat of fusion of ice: L = 3.36 × 105 J kg–1.
Thermal conductivity of ice: K0. = 1.7 W m–1 °C–1.
Formula used:
Rate of amount of heat flowing or heat current is given as:![]()
Here, Δθ is the amount of heat transferred, ΔT is the temperature difference, K is the thermal conductivity of the material, A is the area of cross section of the material and x is the thickness or length of the material.
Also,
Δθ = Q =L× m
Here, Q is the amount of heat absorbed or released, L is the Latent heat and m is the mass of the substance.
And,
Mass = Density × Volume
∴ m = ρ V = ρAl
Here ρ is the density of water, A is the area and l is the length.
(a)
Let change in thickness be Δx,thus rate of change of thickness is:![]()
![]()
![]()
![]()
![]()
Hence, the thickness of the ice increases at a rate of 5.05× 10-7 m/s.
(b)
Consider time required to form thin layer of ice dx is dt.
Using above formulations,
Mass of dx: dm = Adx × ρ
And heat absorbed by the thin layer,
dQ = dm× L
Now, rate of Heat transfer due to thin layer becomes:![]()
![]()
![]()
![]()
Integrating on both sides and setting the limit of ice formed x: 0 to 0.1![]()
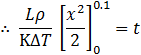
![]()
![]()
![]()
![]()
Hence, it took 27.45 hours to form 10 cm thick ice.