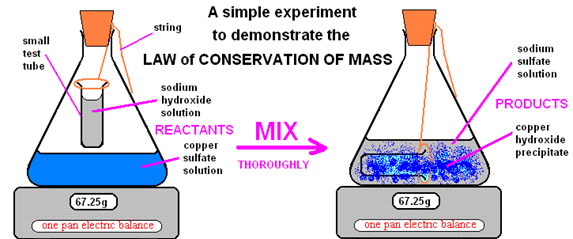
With the help of example, explain the law of conservation of mass.
Law of conservation of mass: This law of states that “atoms are neither created nor destroyed in chemical reaction”. During a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is known as the law of conservation of mass.
This means that the total mass of the products formed in chemical reaction must be equal to the mass of reactants consumed.
For example:
CaCO3 → CaO + CO2
100g 56g 44g
Sum of masses of reactants = Sum of masses of products
1