Consider the de Broglie wavelength of an electron and a proton. Which wavelength is smaller if the two particles have (a) the same speed (b) the same momentum (c) the same energy?
De Broglie wavelength is given by
![]()
Where ![]() =Planck’s constant;
=Planck’s constant; ![]() =mass of the particle;
=mass of the particle; ![]() =momentum and
=momentum and ![]() =velocity of the particle
=velocity of the particle
Mass of electron ![]() kg; mass of proton
kg; mass of proton ![]() kg
kg
(a) Electron and Proton both have same speed
![]()
![]()
The wavelength of proton is smaller than that of electron.
(b) Electron and Proton both have same momentum
![]()
![]()
The wavelength of proton is equal to that of electron.
(c) Electron and Proton have same energy
De Broglie Wavelength is also given by
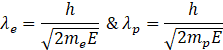
Where ![]() =Energy of the particle
=Energy of the particle
![]()
![]()
The wavelength of proton is smaller than that of electron.
1